Blood, Vol. 110, Issue 1, 171-179, July 1, 2007
The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway
Blood Chen et al. 110: 171
Supplemental materials for: Chen et al
Files in this Data Supplement:
- Figure S1. Study of myosin-IIA function in MK maturation by ES cell differentiation (JPG, 53 KB) -
(A) Methylcellulose colony formation by progenitors derived from Myh9+/− (red) and Myh9−/− (gray) embryoid bodies (EBs) on differentiation day 6. Myh9−/− ES cells show reduced myeloid differentiation and increased formation of secondary EBs. (B) Schematic representation of myosin-IIA constructs used in our studies. (C) Tubulin immunostaining of platelet-like particles released by Myh9+/− ES differentiated MKs, showing the substantial variation in size and shape that precludes application of the model to study defects in platelet form per se. (D) Flow cytometry analysis of platelet-like particles released by wild type or Myh9-transduced fetal liver MKs. PLT, circulating platelets; WT, wild type; GF, GFP-full length Myh9; GX, GFP-R1933X. Particles that are positive for both CD61 and GFP (in GF, GX and GFP) or positive for both CD61 and CD41 (in WT, PLT) were used to back-gate the original scatter plot. The resulting size distributions of such double-positive particles and platelets are shown. The gate drawn to encompass 99.5% of circulating platelets is also applied to each of the other analyses.
- Figure S2. Effects of exogenous myosin-IIA expression on proplatelet morphology (JPG, 93.8 KB) -
(A) Left, Assessment of full length myosin-IIA expression by retroviral transduction of pMIB-MYH9 in Myh9−/− ES cells. Cells surviving blasticidin selection were expanded and lysates from equal numbers of Myh9+/−, Myh9−/− and transduced Myh9−/− ES cells were compared by immunoblotting. Right, myosin-IIA immunoblot analysis to assess exogenous expression of GFP-tagged myosin-IIA rod domain in primary fetal liver-derived MKs. Shown is a representative result for total cell lysates from a culture in which 33% of MKs showed a GFP signal by flow cytometry. Correction of the signal density (NIH Image) for this transduction efficiency indicated ∼7-fold average overexpression compared to endogenous levels of myosin-IIA. (B) Exogenous expression of full length and R1933X in fetal liver MKs. Representative cells are shown, with GFP and phase-contrast images from the same microscopic field; a small area in each panel from the center row is enlarged in the row below to highlight characteristic morphology. Long, beaded projections from MKs expressing GFP contrast with grape-like clusters wrapping near the cell body when MKs express excess full-length myosin-IIA. A few such cells appear in MKs expressing the R1933X myosin-IIA mutant form identified in some patients with the May-Hegglin anomaly, whereas other MKs in the same cultures elaborate normal proplatelets.
- Figure S3. Functional differences between myosin-IIA constructs assessed in HUVEC (JPG, 89.1 KB) -
GFP tagged myosin-IIA constructs were introduced in HUVEC by retroviral infection and their expression followed by green fluorescence. Actin stress fibers were stained by Alexa594-labeled phalloidin (red) and nuclei with DAPI (blue). Full-length myosin-IIA and the R1933X mutant seemed to incorporate normally into actin stress fibers. In contrast, stress fibers were reduced in cells expressing GFP-fused rod domain; neighboring untransduced cells showed intact actin stress fibers (red, arrows).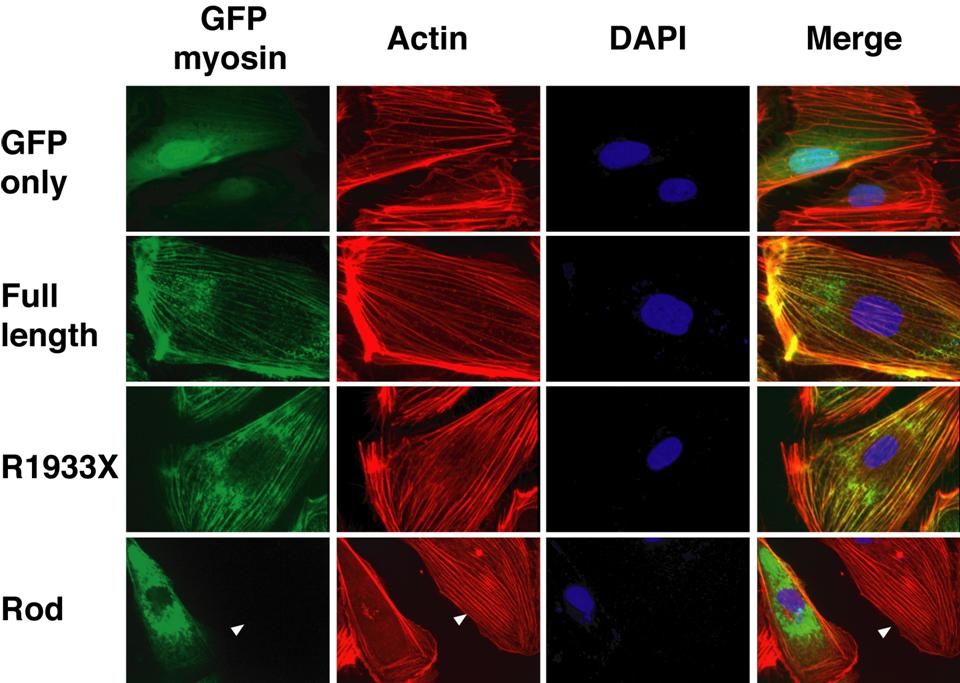
- Figure S4. Regulation of PPF by MLC and Rho (JPG, 52.8 KB) -
(A) Assessment of exogenous MLC expression in fetal liver MKs by immunoblot analysis. In this representative experiment, flow cytometry estimated transduction efficiencies for D18D19-MLC and A18A19-MLC at 44% and 28%, respectively, resulting in average ∼1.7-fold overexpression of each. (B) Rescue of PPF in RhoAv14-expressing MKs by co-expression of A18A19-MLC. Compared to control GFP-transduced cells (arrowhead points to a representative proplatelet), mature RhoAv14-expressing MKs showed greatly reduced PPF. Proplatelets were restored upon double infection with RFP-A18A19-MLC and RhoAV14, as indicated again by arrowheads.
- Figure S5. Regulation of platelet biogenesis by the Rho-ROCK-MLC-myosin pathway (JPG, 26.5 KB) -
A tentative model to illustrate the presumptively competing effects of extracellular factors such as collagen I (activating for Rho) and Sdf-1 (inhibitory toward Rho) on a signaling pathway that converges on myosin IIA-mediated restraints on PPF. Platelet release would be constitutively repressed early in MK ontogeny and enabled in the face of suitable cell maturation, when the Rho-ROCK-myosin pathway is inhibited. Our study helps define the intracellular pathway but candidate ligands and receptors are derived from the published literature or results that require further investigation.
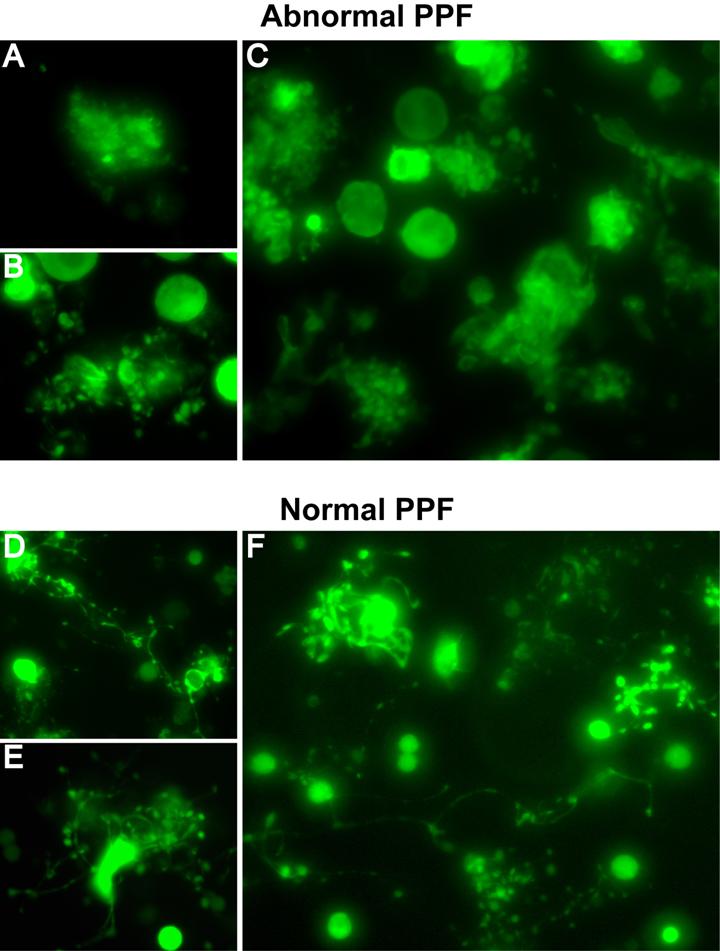
- Figure S6. Morphologic criteria for scoring normal and abnormal PPF (JPG, 59.4 KB) -
(A-C) Representative morphologies of abnormal PPF. A, MK expressing GFP-tagged full length myosin-IIA, showing a ruffled surface without proplatelets. B, MK expressing GFP-tagged wild type MLC, revealing short proplatelets and enlarged apparent diameter resulting from grape-like clusters surrounding the cell body. C, view of a larger microscope field showing multiple GFP-MLC expressing MKs with abnormal PPF similar to those seen in panel B. (D-F) Representative morphologies of normal PPF. D-E, GFP-expressing MKs showing typical long and thin proplatelet filaments with branches. F, view of a larger microscope field with multiple examples of MKs with typical PPF.