Nishimura-Akiyoshi et 10.1073/pnas.0706919104.XXYYYYY103. |

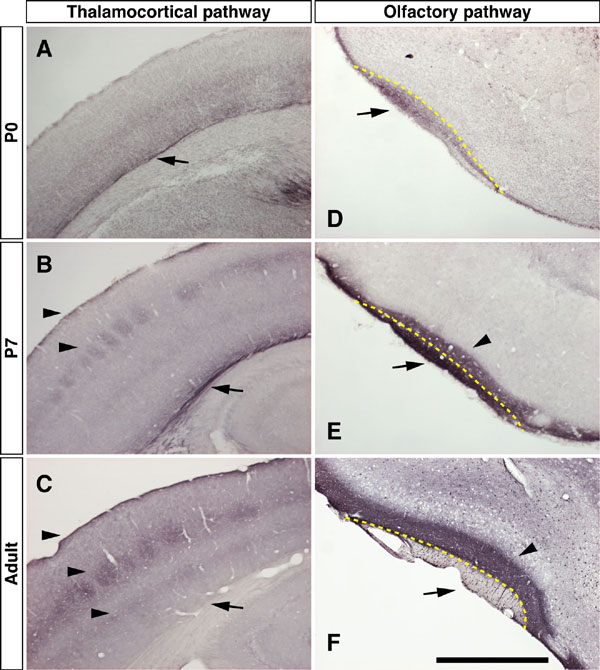
Fig. 6. Axonal localization of netrin-G1 during postnatal development. Immunostaining of brain sections from P0, P7, and adult mice with anti-netrin-G1 antibody. Arrows denote the fasciculated axonal tracts of thalamocortical projection (A-C) and olfactory mitral cells (D-F). Arrowheads indicate the terminal layers of these pathways. In P0 brains, netrin-G1 located these fiber tracts of growing axons. In P7, netrin-G1 immunoreactivity was also detected in the terminal layers of each pathway. In adult brain, netrin-G1 on the axonal tracts was undetectable, and protein distribution was restricted to the axon terminal regions. [Scale bar: 400 mm (A); 800 mm (B and C); 500 mm (D-F).]
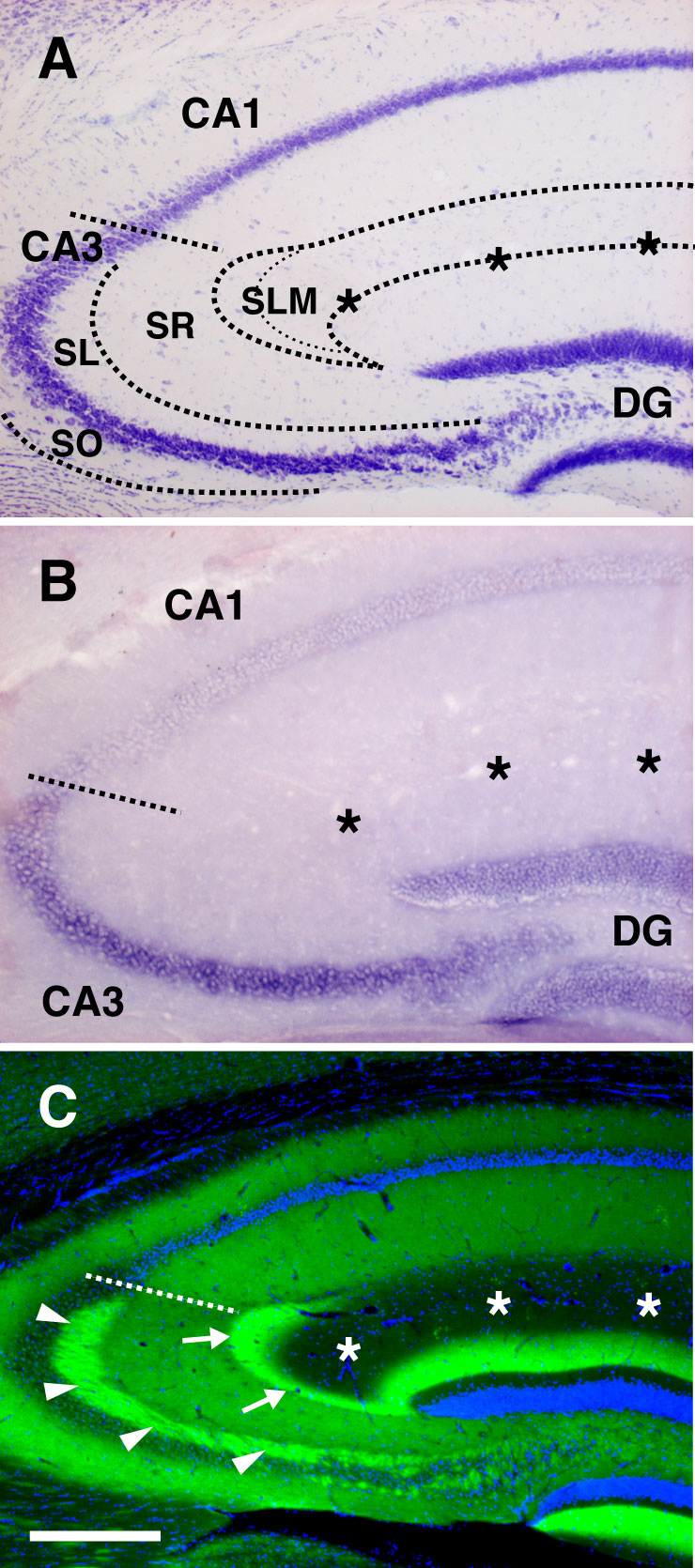
Fig. 7. Laminar distribution of netrin-G2 in the hippocampal CA3 field. (A) Laminar organization of the CA3 field of the hippocampus. (B) In situ hybridization analysis revealed high expression of Ntng2 mRNA in the DG granule cells and CA3 pyramidal neurons, but not in the CA1 neurons. (C) Immunostaining of netrin-G2 in the hippocampus. In the CA3, netrin-G2 proteins are enriched in the stratum lacunosum-moleculare (SLM, the terminal layer of the medial perforant path; arrows), stratum lucidum (SL, the terminal layer of the mossy fibers from the DG; arrowheads), and also stratum radiatum and stratum oriens (SR and OR, the layers innervated by CA3-CA3 associational projections). [Scale bar: 250 mm (A and B); 300 mm (C).]

Fig. 8. No compensatory expression of netrin-Gs in the null mutant mice. (A and B) Northern blot analysis of brain RNA from wild-type (+/+), heterozygous (+/-), and homozygous (-/-) mice of Ntng1 and Ntng2 KO mouse lines with full-length cDNA probes. Coronal brain sections from each genotype were stained with anti-netrin-G1 (C-E) and anti-netrin-G2 antibodies (F-H). There were no detectable transcripts or immunoreactivity observed in the mutant homozygotes. The staining pattern of netrin-G1 and netrin-G2 in Ntng2 KO and Ntng1 KO brain, respectively, did not differ from that in wild-type brain. These data revealed null mutation of the targeted netrin-G and no compensatory expression of the other in each mutant line. (Scale bar: 1 mm.)

Fig. 9. Normal neuroanatomy in netrin-G1 and netrin-G2 mutant mice. (A-C) Cresyl violet staining of adult brain sections. There were no gross abnormalities of brain anatomy in the mutant mice. (D and E) Thalamocortical axons were visualized with the lipophilic tracer 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine (DiI). P4 data are shown here. (F and G) Slices of flat-mounted neocortex were stained by cytochrome oxidase histochemistry, and the barrel patterns in layer IV of somatosensory cortex were visualized. Layer-specific arborization and gross topographic arrangement of thalamocortical projections were normal in Ntng1 KO mice. Lateral (H and I) and medial (J and K) perforant paths were anterogradely labeled with biotinylated dextran amines (BDA). Lamina-restricted termination of axons were observed in Ntng1 KO (I) and Ntng2 KO (K) as in wild-type hippocampus (H and J). Asterisks indicate the hippocampal fissure. (L and M) Lamina-specific projections of the mitral cell axons to the piriform cortex layer Ia were labeled with BDA injection into the olfactory bulb of wild-type and Ntng1 KO mice. [Scale bar: 2.4 mm (A-C); 400 mm (D and E); 1 mm (F and G); 160 mm (H-K); 375 mm (L and M).]

Fig. 10. Density of PSD-95 clusters in netrin-G mutant hippocampus. (A-C) Clusters of PSD-95 in wild-type, Ntng1 KO, and Ntng2 KO hippocampus, detected by immunostaining with anti-PSD-95 antibody. Representative images from the stratum radiatum of CA1 are shown. (Scale bar, 5 mm.) (D) Density of PSD-95 clusters was not different in any layer of hippocampus among the three genotypes. IML, inner molecular layer; MML, middle molecular layer; OML, outer molecular layer; SLM, stratum lacunosum-moleculare; SO, stratum oriens; SR, stratum radiatum. Data are presented as mean ±SEM.
SI Materials and Methods
Expression Vectors, Cell Surface Binding Assay, and BIAcore Analysis.
Full-length coding sequences of mouse NGLs were amplified by RT-PCR from adult mouse brain RNA and subcloned into pECFP-N1 (Clontech, Mountain View, CA). Expression of intact proteins was confirmed by Western blotting with antibodies against NGL-1, NGL-2, or GFP. Two days after transfection into HEK293T cells, myc-tagged netrin-G1a and netrin-G2a proteins (8,9) were added to the culture media for 90 min at room temperature and washed three times with PBS. After fixing the cells with 4% paraformaldehyde in 0.1 M phosphate buffer (PFA/PB), pH 7.4, binding of the tagged proteins was detected by using anti-myc antibody (9E10; Roche Applied Science, Indianapolis, IN).
For BIAcore analysis, extracellular domains of NGLs were ligated into pCMV-Tag vector (Stratagene, La Jolla, CA), resulting in C-terminal FLAG-tagged constructs. Recombinant NGL-ECD-FLAG proteins were produced in HEK293T cells, purified from the culture media on anti-FLAG affinity columns (Sigma-Aldrich, St. Louis, MO), and immobilized onto the surface of sensor chip CM5 by using amine coupling kit (BIAcore). Measurement of the binding of netrin-G1a-myc and netrin-G2a-myc to an NGL-derivatized sensorchip was performed on the BIAcore 2000 biosensor according to the manufacturer's instructions. Netrin-G-myc proteins were serially diluted (37.5-600 nM) in running buffer (PBS/0.005% Tween20). The surface of the sensorchip was regenerated after each injection of analytes with 2M NaCl/PBS/0.5% Tween20.
Histologic Analyses
. Tracing of thalamocortical projections. Brains of postnatal day 3-9 mice were fixed (4% PFA/PB overnight at 4°C) and cut sagittally at the midline, and small crystals of the lipophilic tracer 1, 1'dioctadecyl-3,3,3',3-tetramethylindocarbocyanine (DiI; Molecular Probes, Eugene OR) were implanted into the dorsal thalamus. The tissues were kept in 1% PFA/PB at 37°C for 2-4 weeks. Coronal sections (100 mm) were cut on a microslicer and examined under a fluorescence microscope.
Visualization of barrels (cytochrome oxidase staining
). Brains were fixed with 4% PFA/PB overnight at 4°C and the cortical hemispheres were flattened between glass slides in phosphate buffer overnight at 4°C. Slices (100 mm) were cut tangentially to the pial surface with a microslicer and incubated in the staining solution (50 mg cytochrome C/80 mg DAB/4 g sucrose in 100 ml phosphate buffer) at 37°C for 7 h.
Tracing of perforant paths and olfactory projection
. Adult mice were anesthetized and a 10% solution of biotinylated dextran amines (BDA-10,000; Molecular Probes, Eugene, OR) was injected into the lateral or medial entorhinal cortex, or olfactory bulb by using a stereotaxic apparatus (0.2 ml per injection). Four days after the injection, brains were fixed with 4% PFA/PB and serial coronal sections (40 mm) were cut on a freezing microtome. One-in-three sections were incubated with a solution of avidin and biotinylated HRP complexes, and stained with diaminobenzidine/nickel substrate solution (Vector Laboratories, Burlingame, CA).
Quantitative analysis of PSD-95 clusters
. Brains were removed and frozen immediately after perfusing with saline. Fresh frozen sections (8 mm) were mounted on glass slides, fixed with cold methanol, and incubated with anti-PSD-95 antibody (Clone 7E3-1B8; 1:1000; Affinity BioReagents, Golden, CO). Fluorescence images were taken from the middle of each layer of the hippocampus by using a confocal laser scanning microscopy with a 100´ objective lens and 4´ digital zoom at the same acquisition settings. The amount of clusters was counted using Image-Pro Plus (Media Cybernetics, Silver Spring, MD).