Giordani et al. 10.1073/pnas.0611299104. |
Fig. 6. (I) Whole-mount in situ hybridization with Myf5 (a, c, and e) and Pax3 (b, d, and f) probes on wild type (a and b), Six1-/- (c and d), and Six1-/-Six4-/+ (e and f) embryos at E11.5. Arrowheads at the forelimb (FL) and hindlimb (HL) levels pinpoint the signals where they are detectable. (II) At E11.5, 100-mm vibratome cross-sections of Myf5 and Pax3 in situ hybridization (ISH) in wild-type and Six1-/- embryos at the HL and FL levels and in Six1-/- Six4-/+ embryos at the HL level. Arrowheads show a weak signal for Myf5 transcripts in the ventral aspect of the limbs in Six1-/- and Six1-/- Six4-/+ embryos and for Pax3 transcripts at the FL level of Six1-/- embryos, and at the HL level of Six1-/- Six4-/+ embryos. Note the absence of signal for Myf5 in Six1-/- FL. (III) At E12.5, 100-mm vibratome sagital cross-sections of FL and frontal cross-sections of distal hindlimbs (DHL) or of proximal hindlimbs (PHL) from whole-mount in situ hybridization (Fig. 1C) performed with Myf5 or MyoD probes in wild-type or Six1-/- embryos. Note that the embryos have been sectioned sagitally for the ISH experiment, so that one half of the embryo has been hybridized with a Myf5 probe and the other half with a MyoD probe to compare the expression pattern of Myf5 and MyoD on the same embryo. In the Six1-/- FL most MyoD and Myf5 expression is lost except in the posterior aspect of the limb (arrowheads). In the DHL cells with Myf5 and MyoD transcripts become detectable at 500 mm from the tip of the wild-type limb, whereas in Six1-/- embryos cells with MyoD transcripts become detectable at 600 mm and with Myf5 transcripts at 900 mm from the tip of the limbs, reflecting a greater decrease of the Myf5 signal in distal myogenic cells as compared with MyoD. This illustrates that absence of Six1 precludes early full activation of Myf5 in myogenic progenitors. In the PHL, Myf5 and MyoD transcripts can be detected dorsally.
Fig. 7. (A) Gel mobility shift assays (GMSA) showing the ability of Six1 and Six4 proteins to recognize the Myf5 MEF3 element. In vitro translated Six1 or Six4 proteins were incubated with the Myf5 oligonucleotide with the MEF3 site in the presence or absence of Six1 or Six4 antibodies, as indicated. (B) Binding of Pax3 and Six1 to the larger Myf5 regulatory element that contains their respective binding sites was examined by GMSA experiments. Individually each protein is able to bind to the fragment (lanes 3 and 5), and this is confirmed by the addition of Pax3 (lane 4) and Six1 (lane 6) antibodies, which supershift or disrupt the DNA--protein complex, respectively. The Pax3 binding site competes Pax3 binding to the larger sequence as expected (lanes 10-13). Pax3 binds (lanes 14-17) equally well when the Six1 site is mutated and shows a similar reduction when competed with this sequence (lanes 14-17), thus demonstrating that the Six1 site is not implicated in Pax3 binding. Mutations in this site therefore reflect a role for Six1 and not Pax3 in activating the Myf5 enhancer. When Pax3 and Six1 are present together, a larger complex is formed that may correspond (up arrow in lane 7) to Pax3 and Six1 protein binding to the same fragment. It is shifted by the Pax3 antibody (lane 8) but not by the Six1 antibodies, which may be due to masking of Six1 epitopes in a Pax3/Six1 complex. Traces of this complex with Pax3 alone probably reflect endogenous Six activity in the reticulocyte lysate as indicated by the Six1 band. Lanes 1 and 3-13, probe 119/120 (single line); lanes 2 and 14-17, probe 123/124 (double line); lanes 1 and 2, crude reticulocyte lysate; lane 3, Pax3; lane 4, Pax3 + Pax3Ab; lane 5, Six1; lane 6, Six1 + Six1Ab; lane 7, Pax3 + Six1; lane 8, Pax3 + Six1 + Pax3Ab; lane 9, Pax3 + Six1 + Six1Ab; lane 10, Pax3 + Six1 + 10 molar excess of oligo 135/136; lane 11, Pax3 + Six1 + 25 molar excess of oligo 135/136; lane 12, Pax3 + Six1 + 50 molar excess of oligo 135/136; lane 13, Pax3 + Six1 + 250 molar excess of oligo 135/136; lane 14, Pax3 + Six1 + 10 molar excess of oligo 135/136; lane 15, Pax3 + Six1 + 25 molar excess of cold Pax3BS oligo 135/136; lane 16, Pax3 + Six1 + 50 molar excess of oligo 135/136; lane 17, Pax3 + Six1 + 250 molar excess of oligo 135/136.

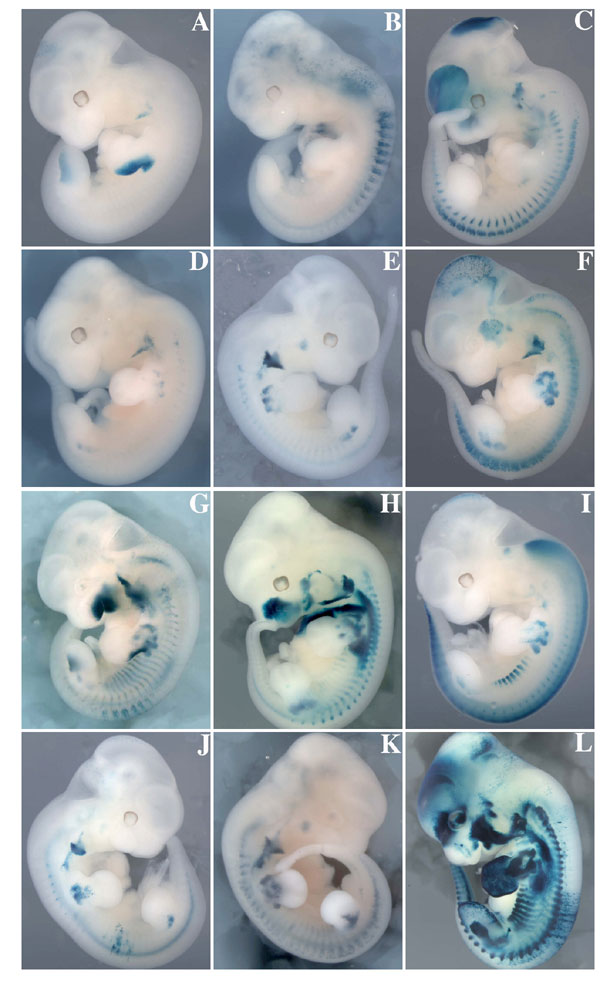
Fig. 8. X-gal staining of transgenic embryos with the -58/-57SixMutbaMyf5nlacZ transgene at E11.5. A range of expression profiles was obtained, and the embryos are shown from the weakest to the strongest expression profile observed. Three were negative in the limb buds (A-C), and six were negative in the somites (A, B, D-F, and J). In some of the latter (F), residual expression in the trunk is in the dorsal root ganglia. Note that in 11 of 12 embryos (A-K), full proximo-distal limb expression of the transgene is compromised. One embryo (L) shows extensive ectopic expression of the transgene, indicating an integration site effect. The branchial arch element provides a positive control in most embryos.
Fig. 9. Schematic representation of the genetic hierarchy operating during limb muscle development. It has been shown that Pax3 expression in the hypaxial dermomyotome is under the control of Six1/Six4 proteins (1) and that Pax3 directly controls Myf5 expression (2). We now show that Six proteins directly control the limb expression of Myf5 (this study). Six proteins also directly control Myogenin (3) and muscles genes expressed in differentiated myofibres such as MCK (4) or aldolase A (5). Thus, Six proteins participate in each step of limb myogenesis, together with Pax3 to activate Myf5, with Myf5 or MyoD to activate Myogenin, and with Myogenin to activate muscle genes specific to the differentiated fiber. Dashed arrows represent other inputs that regulate this cascade.
1. Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller M, Hamard G, Maire P (2005) Development (Cambridge, UK) 132:2235-2249.
2. Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham M (2006) Genes Dev 20:2450-2464.
3. Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P (1998) Proc Natl Acad Sci USA 95:14220-14225.
4. Himeda CL, Ranish JA, Angello JC, Maire P, Aebersold R, Hauschka SD (2004) Mol Cell Biol 24:2132-2143.
5. Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, et al. (2004) Mol Cell Biol 24:6253-6267.
SI Text
Statistical Analysis.
Statistical analysis was performed by using Statview 5.0 software (SAS Institute). All values are given as means ± SEM. Homogeneity of variance between groups was assessed by using the Bartlett test. Because variances were comparable, the effect of the MEF3 mutation on each transfection experiment was determined by using a one-way ANOVA. The effect of Six1 cotransfection was assessed by a Student-Newman-Keuls test for multiple mean comparisons.
Metamorph Software.
To quantify the relative extent of Myf5+/Pax3+ areas, we performed serial vibratome sections through the limb buds of embryos in which in toto in situ hybridization (ISH) had been performed with Pax3 and Myf5 probes. We calculated the cumulated areas of expression of both genes over the entire length of the limb buds using Metamorph software. The areas of Pax3 and Myf5 gene expression were calculated after having manually delimited surfaces of ISH positive staining for each gene on each vibratome section.
Immunohistochemistry.
E10.5 embryos were fixed in 4% paraformaldehyde (PFA) for 2 h at 4°C, washed twice for 4 h in 1´ PBS. Fixed embryos were incubated overnight in 20% sucrose before being frozen in isopentane and sectioned (20 mm). Dried sections were incubated for 20 min in 1´ PBS/0.1% Triton X-100, blocked for 1 h in saturation solution (1´ PBS/1.6% goat serum/10% BSA/0.1% Triton X-100), incubated with Six1 antibodies (1) (1/1,000), and revealed as recommended by the manufacturer (TSA fluorescent system; PerkinElmer). Sections were further incubated for 2 h with Pax3 antibodies (1/2,000) in saturation solution. After three washes in PBT (1´ PBS/0.1% Tween 20), slides were incubated for 1 h with secondary antibodies (1/600 anti-mouse-Cy3; Jackson Laboratories) and washed in PBT before mounting in Vectashield. E11.5 embryos were prepared as described previously (2). Embryos were frozen in isopentane and sectioned (14 mm). Immunochemistry on sections was performed as described (2) with the following modification: after incubation with the secondary antibodies, slides were washed twice in 1´PBS for 10 min and once in 4´ PBS for 10 min. Primary antibodies used were polyclonal rabbit anti-Myf5 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:2,000 and monoclonal mouse anti-Pax3 (kindly provided by M. Bronner-Fraser, Pasadena, CA) diluted 1:100. Secondary antibodies used were Alexa Fluor 546-conjugated goat anti-rabbit and Alexa Fluor 488-conjugated goat anti-mouse (Molecular Probes), both diluted 1:500.
Primers used for chromatin immunoprecipitation experiments were as follows: Myf5F, 5'-AGG CAT GAC TAA TTG CAT GGT AAC TGG; Myf5R, 5'-CTC ATA ATG ATA TGG TTT TAA GCC CC; -200 kb Myf5F, 5'-GTG TGT CAG TGC ATA GCC TAA; -200 kb Myf5R, 5'-AGG AAG AGC TTG ATG GAC CAA; H4F, 5'-GAC ACC GCA TGC AAA GAA TAG CTG; H4R, 5'-CTT TCC CAA GGC CTT TAC CAC C.
Plasmid Constructions.
Mutagenesis was performed with the QuikChange Site-Directed Mutagenesis (Stratagene, La Jolla, CA), by using as a matrix a plasmid where the -58/-57-kb fragment was subcloned into the pGEMt-easy vector (Promega). The -58/-57SixMut fragment was released from the pGEMt-easy vector with HindIII/XhoI and subcloned into pbaMyf5nlacZ (3). The primer used for mutagenesis was 5'-GAC TAA TTG CAT GGT AAC TGC GCA AAT GCT TTC TCT CTC-3'.
Generation and Analysis of Transient Transgenic Embryos.
Plasmid fragment purification was carried out as described (4). Transgenic mice were generated by microinjection of purified plasmid DNA into fertilized (C57BL/6J´SJL) F2 eggs at a concentration of »1 ng/ml using standard techniques. Injected eggs were reimplanted the same day or the day after the injection into outbred pseudopregnant foster mothers. Transient transgenic embryos were dated taking the day of reimplantation into the pseudopregnant foster mothers as E0.5. Embryos were dissected in PBS, fixed in 4% paraformaldehyde for 15 min, rinsed three times in PBS, and stained in X-gal solution (5) at 37°C overnight. DNA was prepared from Xgal negative embryos and analyzed by PCR using nlacZ primers.
In Situ
Hybridization and GMSA.Whole-mount in situ hybridization experiments with Pax3, Myf5, and MyoD probes were performed as described previously (6), with a minimum of three embryos of each genotype for each probe tested. One-hundred-micrometer vibratome sections were analyzed essentially as described in ref. 6.
Gel mobility shift assays (GMSA) were carried out with Six1 and Six4 full-length mouse cDNA cloned into the pCR3 vector (Clontech) (1) vector or with Pax3 cDNA cloned into the pCDNA3.1+ (Invitrogen) (generous gift from F. Relaix). Recombinant proteins were obtained with a T7 transcription/translation kit (Promega). Labeled double-stranded DNA containing part of the Myf5 145-bp sequence with the MEF3 site (sequence 5'-AGAAAGCATTTCTCCAGTTACCATGCAAT) or the mutant MEF3 site (5'-AGAAAGCATTTgcgAGTTACCATGCAAT), Pax3 and MEF3 sites 119/120 probe (5'-GATATAAATCATAAAGGCATGACTAATTGCATGGTAACTGGAGAAATGCTTTC-3'), and Pax3 and mutant MEF3 sites 123/124 probe (5'-GATATAAATCATAAAGGCATGACTAATTGCATGGTAACTGcgcAAATGCTTTC-3') were incubated with recombinant proteins and GMSA performed as described previously (1). Competition experiments were performed with double-stranded DNA containing only the Pax3 binding site (Pax3BS) 135/136 (5'-AATCATAAAGGCATGACTAATTGCATGG-3').
1. Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P (1998) Proc Natl Acad Sci USA 95:14220-14225.
2. Bajanca F, Luz M, Duxson MJ, Thorsteinsdottir S (2004) Dev Dyn 231:402-415.
3. Hadchouel J, Carvajal JJ, Daubas P, Bajard L, Chang T, Rocancourt D, Cox D, Summerbell D, Tajbakhsh S, Rigby PW, et al. (2003) Development (Cambridge, UK) 130:3415-3426.
4. Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M (1995) J Cell Biol 129:383-396.
5. Tajbakhsh S, Buckingham ME (1994) Proc Natl Acad Sci USA 91:747-751.
6. Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller M, Hamard G, Maire P (2005) Development (Cambridge, UK) 132:2235-2249.