Xie et al. 10.1073/pnas.0707909104. |

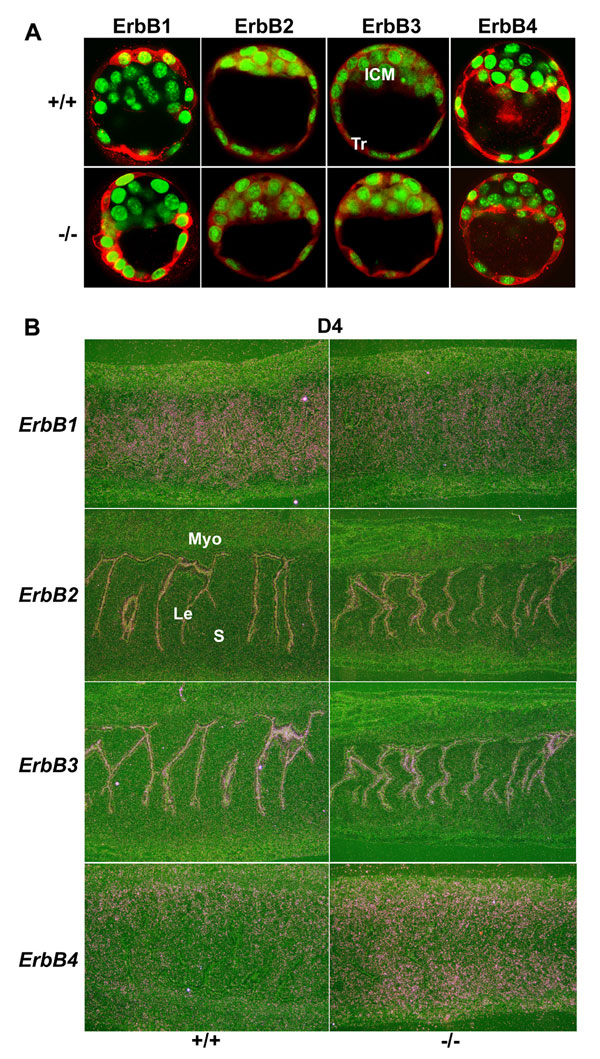
Fig. 6. Comparable expression patterns of ErbB receptors in WT and Hbegf-/- day 4 embryos and uteri. (A) Immunofluorescence staining shows ErbB receptor expression in Wt and mutant blastocysts. ICM, inner cell mass; Tr, trophoblast cells. (B) In situ hybridization showing expression of ErbB receptors in day 4 WT and mutant uteri (´40). Le, luminal epithelium; S, stroma; Myo, myometrium.
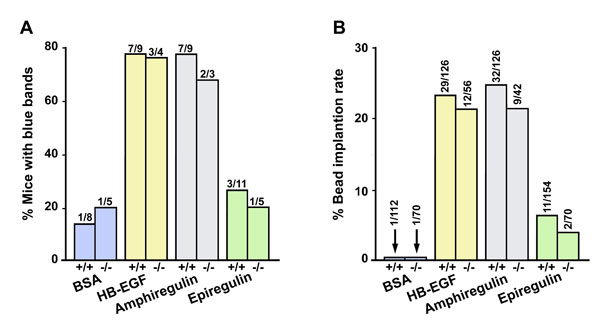
Fig. 7. HB-EGF and amphiregulin-soaked beads induce implantation-like blue reaction in WT and Hbegf-/- mice. Beads soaked with BSA, HB-EGF, amphiregulin, or epiregulin proteins were transferred into day 4 pseudopregnant WT and mutant uteri. Implantation was examined by the blue dye method 24 h after transfer. (A) Percentage of bead-transferred recipients showing blue reaction. Numbers above bars indicate number of recipients with blue bands per the total number of recipients. (B) Implantation rates of transferred beads in WT and mutant recipients. Numbers above the bars indicate number of blue bands per the total number of beads transferred.

Fig. 8. Role of HB-EGF signaling in ovarian steroid hormone synthesis. (A) Immunolocalization of HB-EGF and ErbB1-4 in WT ovaries on days 1 and 4 of pregnancy (´40). (B and C) Detection of P450scc, 3b-HSD, and P450 aromatase in WT and Hbegf-/- day 4 ovaries by immunohistochemistry (´40) and Western blotting analysis. Actin acts as a control. cl, corpus luteum; f, follicle.
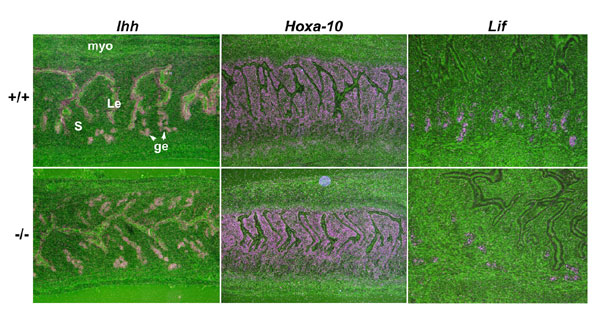
Fig. 9. Expression of progesterone and estrogen target genes in Hbegf null mice on day 4 of pregnancy. In situ hybridization analysis revealed comparable expression of Ihh and Hoxa-10, known progesterone-target genes, as well as Lif, an estrogen-responsive gene, in WT and Hbegf-/- uteri on day 4 of pregnancy (´40). Le, luminal epithelium; s, stroma; ge, glandular epithelium; Myo, myometrium.
SI Methods
Ovulation, Fertilization, Attachment Reaction, Delayed Implantation, and Blastocyst Transfer. F
emale mice were mated with fertile or vasectomized male mice of the same genotype to induce pregnancy or pseudopregnancy, respectively (day 1 = vaginal plug). To examine ovulation and fertilization, mice were killed on day 2 of pregnancy and oviducts were flushed with Whitten's medium to recover eggs and embryos. To examine the attachment reaction, pregnant WT or Hbegf-/- mice were killed at 2000 or 2400h on day 4 or at 0800h on day 5 of pregnancy. Implantation sites were visualized by i.v. injection of Chicago blue dye solution (1). Uteri of mice without implantation sites were flushed with Whitten's medium to recover unimplanted blastocysts. To induce delayed implantation, pregnant mice were ovariectomized on the morning of day 4 (0900 h) and the condition maintained by daily injections of P4 (2 mg/mouse) from day 5 until the day of sacrifice. Blastocyst attachment was initiated in P4-primed delayed implanting pregnant mice by giving an injection of E2 (3 or 25 ng/mouse). For reciprocal embryo transfer experiments, day 4 WT or Hbegf-/- blastocysts were transferred into the uteri of WT or Hbegf-/- pseudopregnant recipients on day 4, and implantation was examined by blue dye method 24 h after transfer. For embryo transfer experiments in delayed implanting mice, day 4 WT blastocysts were transferred into P4-primed delayed WT or Hbegf-/- recipients on day 7 of pseudopregnancy. Immediately after transfer, recipients received an E2 injection (3 or 25 ng/mouse, sc) and implantation rates were examined 24 h later.Growth Factor-Soaked Bead Transfer. M
ating females with vasectomized males induced pseudopregnancy. Affi-Gel Blue beads (100-200 mesh; Bio-Rad) of about the size of a blastocyst were incubated with HB-EGF, amphiregulin, epiregulin (200 ng/ml), or similar concentrations of BSA in 20 ml PBS at 37°C for 1 h as described (2). Loaded beads (seven beads per horn) were transferred into WT or Hbegf-/- uteri on day 4 of pseudopregnancy. All recipients were killed 24 h after transfer, and the number of blue bands was recorded after blue dye injection. All growth factors were purchased from R & D.Immunostaining. I
mmunolocalization of HB-EGF and ErbB receptors in the ovary was performed in formalin-fixed paraffin embedded sections using specific antibodies to HB-EGF (R & D), ErbB1 (Santa Cruz Biotechnology), ErbB2 (R&D), ErbB3 (Santa Cruz), ErbB4 (Santa Cruz), P450scc (Chemicon), 3b-HSD (a gift from Dr. J. Ian Mason at the University of Edinburgh) and P450Arom (Affinity BioReagents), respectively. A Histostain-Plus (DAB) kit (Invitrogen) was used to visualize the antigen as described (3). Immunofluorescence staining of ErbB receptors in day 4 blastocysts was performed as we described (4). Secondary antibodies conjugated with TRITC dye (Jackson ImmunoResearch) were used to detect fluorescence signals. SYTO13 green fluorescence dye (Invitrogen) was used for nuclear staining. Fluorescence images were captured under a Zeiss LSM 510 confocal laser microscope.Western Blotting. P
rotein extraction and Western blotting were conducted as described (5). Antibodies to P450scc, 3b-HSD and P450Arom were used. Bands were visualized by using an ECL kit.1. Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK (1994) Development (Cambridge, UK) 120:1071-83.
2. Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL (2001) Proc Natl Acad Sci USA 98:1047-52.
3. Chakraborty I, Das SK, Wang J, Dey SK (1996) J Mol Endocrinol 16:107-22.
4. Wang X, Wang H, Matsumoto H, Roy SK, Das SK, Paria BC (2002) Development (Cambridge, UK) 129:4125-34.
5. Scherle PA, Ma W, Lim H, Dey SK, Trzaskos JM (2000) J Biol Chem 275:37086-92.