Dupuis et al. 10.1073/pnas.0703842105. |

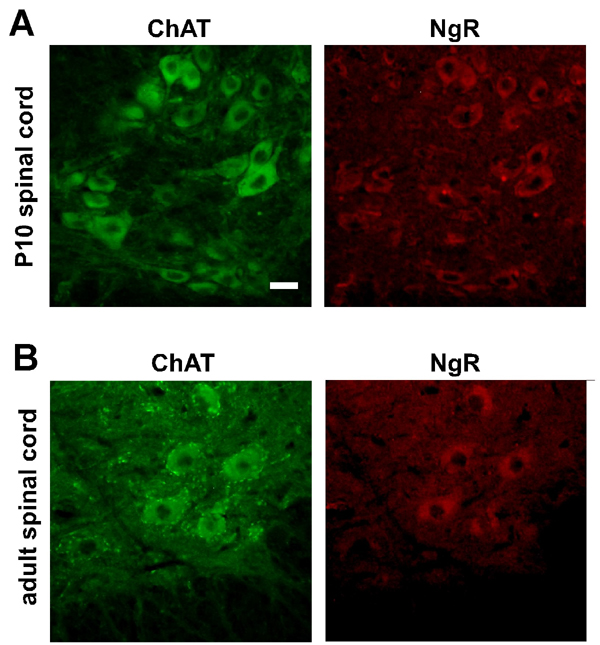
Fig. 6. NgR is expressed in motor neurons throughout lifespan. (A and B) Photomicrographs showing ChAT (green) and NgR (red) immunoreactivity in the lumbar spinal cord of postnatal (P10, A) and adult (B) mice. Scale bar: 25 mm.
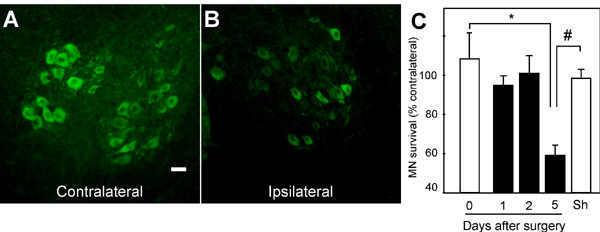
Fig. 7. Motor neurons degenerate following neonatal sciatic nerve axotomy. (A and B) Photomicrographs showing ChAT immunoreactivity in the lumbar spinal cord of neonate mice after 5-days postaxotomy. The sides contralateral (A) and ipsilateral (B) to the lesion are shown. Scale bar: 25 mm. (C) Time-course of the survival rates of ChAT-positive motor neurons after sciatic nerve axotomy in neonate mice. Sham-operated (Sh) animals served as control. Data are presented as percentages of motor neurons in the corresponding side contralateral to the lesion. Note that motor neuron death appears significant 5 days after axotomy, and hence this time point was used in later experiments (* P < 0.05 vs. 0-day; # P < 0.05 vs. 5-day; n = 3 mice per group).

Fig. 8. Pep4 and NEP1-40 cross the blood brain barrier of neonate animals but do not affect motor neuron survival under normal conditions. (A) Photomicrographs representing nuclear Hoechst (Left) or avidin-FITC (Right) labeling in the lumbar spinal cord of neonate mice injected either with vehicle, biotinylated Pep4 or biotinylated NEP1-40. Scale bar: 25 mm. (B) Absolute numbers of motor neurons in the lumbar spinal cord of neonate mice after ChAT immunoreactivity (Left) or toluidine blue staining (Right). In the latter case, only those cells with a cross-sectional area ³1,000 mm2 were considered as motor neurons. Animals were treated during 5 days with vehicle, or 11.6 mg/kg body mass Pep4 or NEP1-40 (P > 0.05; n = 9-11 mice per group).
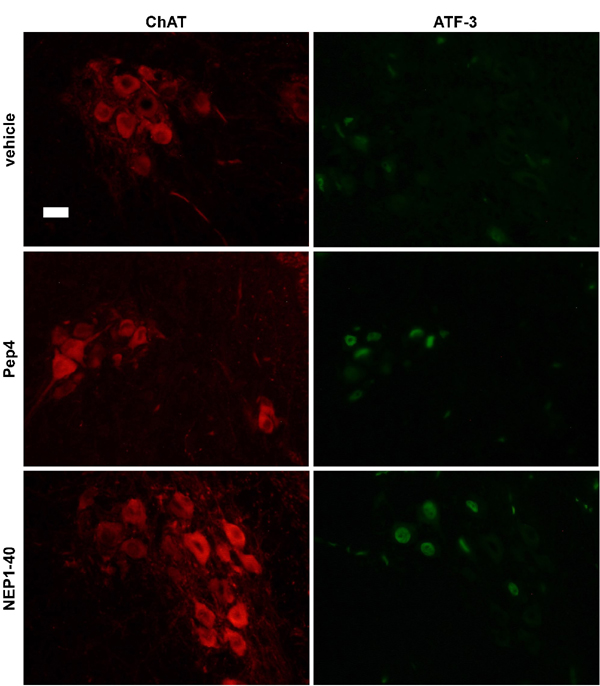
Fig. 9. The neuroprotection offered by Pep4 and NEP1-40 following sciatic nerve axotomy in neonate mice is accompanied by up-regulation of ATF3. Photomicrographs showing ChAT (Left) and ATF3 (Right) immunoreactivity in the lumbar spinal cord of neonate mice. The sciatic nerve was axotomized and animals were treated during 5 days with vehicle, or 11.6 mg/kg body mass Pep4 or NEP1-40. Note that many of the ChAT-positive motor neurons after Pep4 or NEP1-40 administration are also labeled for ATF3, in contrast to what is observed in the control condition. Scale bar: 25 mm.

Fig. 10. GDNF deprivation after 24 h of culture under normal survival conditions does not induce motor neuron death. Scheme representing the experimental setup of motor neuron (MN) cultures maintained in the absence or presence of GDNF during 24 or 72 h. Note that GDNF deprivation after the first 24 h of culture does not affect motor neuron survival, whereas complete removal of GDNF from the culture medium induces about 40% of cell death, as compared to the control condition when GDNF is present for 72 h. These findings validate the choice of using PI-PLC after the first 24 h of culture to remove GPI-anchored proteins from the extracellular membrane (* P < 0.05 vs. GDNF; # P < 0.05 vs. 24h-GDNF; n = 2 independent experiments).
Table 1. PCR primers
cDNA | Forward | Reverse |
P75NTR | gtggaacagctgcaaacaaa | ctctacctcctcacgcttgg |
NgR | tgcagccacaggatagtgag | ctggagggtagcaacaccat |
TAJ/TROY | attccctcaatcccgaaaac | gtcctttgagcatcctgagc |
LINGO-1 | gcctcaacctgacatcccta | ttgccagagacattgagcac |
AChR | CCACAGACTCAGGGGAGAAG | AACGGTGGTGTGTGTTGATG |
Myogenin | CACTCCCTTACGTCCATCGT | CAGGGCACTCATGTCTCTCA |
Musk | ttcagcgggactgagaaact | tgtcttccacgctcagaatc |
18S | GCCATGCATGTCTAAGTACGC | CTGATAAATGCACGCATCC |
SI Text
Cell Culture.
Motor neuron cultures were prepared from rat embryonic (E15) spinal cords by metrizamide gradient centrifugation and IgG192 anti-p75NTR antibody immunopanning as described (1). Primary astrocyte cultures were prepared from the spinal cords of rats aged 1-2 days according to previous studies (2). For coculture experiments, purified motor neurons were plated on rat astrocyte monolayers at a density of 300 cells/cm2 and maintained as indicated (1). In all cases, survival control conditions were considered in the presence of 1 ng/ml GDNF. To remove GPI-anchored proteins, motor neurons were maintained in the presence of GDNF during the first 24 h of culture, and then pretreated with 0.04 units/ml PI-PLC (Sigma) before adding the test substances. Under these experimental conditions, the suspected PI-PLC-mediated removal of the neurotrophic effect of GDNF did not appear to affect overall motor neuron survival (SI Fig. 10). To induce motor neuron death, cultures were treated with 100 ng/ml mouse NGF (2.5S; Harlan) either in the presence of 10 mM NOC-18 or cocultured with astrocytes as a means to generate low steady state concentrations of NO (1). To assess the death-promoting effect of spinal cord extracts, lumbar cords were dissected from symptomatic SOD1(G93A) mice or nontransgenic (wild-type) littermates (strain B6SJL-TgN(SOD1-G93A)1Gur; Jackson Laboratory). Lysates were prepared as previously described and added to motor neuron cultures to reach a final protein concentration of 0.5 mg/ml in the presence of 10 mM NOC-18 (1). Pep4 (ELVQKYSNSALGHVNSTIKELRRLF) and NEP1-40 (RIYKGVIQAIQKSDEGHPFRAYLESEVAISEELVQKYS) derived from Nogo and Pep4 (EAFHNYMNAAMVHVNKALKLIIRLF) and NEP1-40 (RVYKSVIQAVQKSEEGHPF-KAYLDVDITLSSEAFHNYM) derived from RTN3 were synthesized by Millegen and used at concentrations indicated in the figure legends. Soluble NgR (R&D Systems) was used at 27 nM (2 mg/ml).Immunohistochemistry and Western Blot.
Lumbar spinal cords from embryonic, neonate and adult mice were carefully dissected, fixed in 4% PBS-buffered paraformaldehyde and cut into 10-mm sections with a cryostat. ChAT immunoreactivity was detected with goat anti-ChAT (Chemicon International) diluted 1/50 and FITC-coupled donkey anti-goat IgG diluted 1/500 (Jackson Immunoresearch Laboratories). ATF3 immunoreactivity was detected using anti-ATF3 sc-188 (Santa Cruz). NgR immunoreactivity was detected using rabbit anti-NgR (R&D Systems) diluted 1/200 followed by TRITC-coupled donkey anti-rabbit IgG diluted 1/500 (Rockland Immunochemical). Biotinylated peptides were detected using avidin-FITC (Jackson Immunoresearch Laboratories) diluted 1/500. Cultured motor neurons were co-immunostained with mouse anti-p75NTR (R&D Systems) diluted 1/100 and rabbit anti-NgR diluted 1/200, followed by FITC-coupled anti-mouse IgG diluted 1/1,000 and TRITC-coupled donkey anti-rabbit IgG diluted 1/500, respectively.For Western blot, cells were homogenized in PBS containing 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS and 1% protease inhibitor mixture. Homogenates were boiled for 5 min and sonicated for 20 seconds. After centrifugation (15,000 rpm for 20 min at 4°C), 100 mg total protein, according to the bicinchoninic acid assay, were separated on a 10% SDS-polyacrylamide gel. Separated proteins were electrotransferred to nitrocellulose membranes and submitted to reversible staining with 0.1% Ponceau S in 5% acetic acid to ensure homogeneous transfer. Immunostaining was performed with rat anti-NgR antiserum (R&D Systems) and horseradish peroxidase-conjugated goat anti-rat IgG (Pierce) diluted 1/2,000. To ensure that equal amounts of protein were loaded, actin immunoreactivity was determined under the same conditions as described, using a monoclonal antibody to actin (kindly provided by Dr. D. Aunis, Centre de Neurochimie, Strasbourg, France) diluted 1/10 and horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) diluted 1/5,000. Membranes were developed by enhanced chemiluminescence detection.
RT-PCR.
Two micrograms of total RNA extracted from either cultured rat embryonic motor neurons, NSC34 cells, lumbar spinal cord, or hindlimb skeletal muscles was reverse-transcribed by using 200 U of MoMuLV reverse transcriptase and 0.5 mg of random primers (Promega) by a standard procedure. After PCR amplification, products were separated on a 2% agarose gel and visualized by ethidium bromide staining. We used 18S rRNA levels as internal controls. PCR primers are described in SI Table 1.1. Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, Estevez AG, Barbeito L (2004) Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem 89:464-473.
2. Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L (2002) Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res 67:21-29.