 , [S1]
, [S1]Zanchetta et al. 10.1073/pnas.0711319105. |
Fig. 7. Concentration profiles obtained from fluorescence measurements, as described in SI Material and Methods, at different times few minutes after the contact: t = 0, black; t = 30 s, navy; t = 60 s, blue; t = 120 s, cyan; t = 180 s, green; t = 240 s, orange; and t = 300, red.
Fig. 8. The first (t = 0, black squares) and last (t = 300 s, red circles) profile of SI Fig. 7 are shown, together with their fits with Eq. S1 (thin continuous lines).
Fig. 9. Polarized microscopy images of different stages of the phase separation, nucleation, and growth of liquid crystalline domains of a sample with a 16-bp self-complementary sequence mixed with ssDNA sequences (SC-MIX). An image of the sample before heating is shown in a. The cell was heated and kept at high temperature for about 30 min and then rapidly cooled to 25°C. After a lag time of about 10 min, some nuclei appear (b); then after few minutes, the number of nuclei saturates and their size grows until reaching an apparent steady state (c-f). However, on a much bigger time scale (days), the size of the nuclei keeps growing (see text and g). (Scale bars: 50 mm.) (a) Before heating. (b) t = 900 s (after keeping at high temperature and cooling to 25°C). (c) t = 1,920 s. (d) t = 3,000 s. (e) t = 4,800 s. (f) t = 14,700 s. (g) t = 345,600 s.
Fig. 10. Polarized microscopy images of textures in the final stage of the growth process shown in SI Fig. 9. Focal conic, fan-shaped domains reveal the liquid crystalline nature of most of the droplets. (Scale bars: 30 mm.)
SI Materials and Methods
Determination of Diffusion Coefficient
. An independent estimate of the diffusion coefficient of a short duplex in a ssDNA pool was obtained by following the diffusion of A sequence (GGAGTTTTGAGG) in a 1:10 mixture with B sequences (CCTCAAAACTCC) after a contact with a sample of only B sequences at the same concentration. Approximately 2.5% of A was tagged at 5' with fluorescein. The B solution was introduced by capillarity to completely fill a 2-mm-thick homemade cell, and the A solution was put in contact with it on the edge of the cell. The fluorescence signal and its time evolution inside the cell were monitored with fluorescence microscopy imaging. Given the unbalanced concentration of the two strands, we can assume that almost all of the A sequences are in the form of helices; thus, the measured fluorescence intensity is proportional to the concentration c(x, t) of duplexes. The tail of the fluorescence profile (far enough from the edge to exclude evaporation effects) at different times is fitted by a solution of the diffusion equation for two semi-infinite bodies with a "step" initial condition: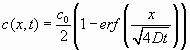 , [S1]
, [S1]
where c0 is the initial concentration of A. From the fitting, the diffusion coefficient D is obtained.