Blood, Vol. 111, Issue 5, 2704-2713, March 1, 2008
TLR9 engagement on CD4 T lymphocytes represses  -radiation–induced apoptosis through activation of checkpoint kinase response elements
-radiation–induced apoptosis through activation of checkpoint kinase response elements
Blood Zheng et al. 111: 2704
Supplemental materials for: Zheng et al
Files in this Data Supplement:
- Figure S1. TLR9 expression is upregulated following T-cell activation (JPG, 62 KB) -
CD4 T-cells were purified by negative selection followed by positive selection using magnetic beads. T cells per well were activated with plate-bound anti-CD3 and CD28 antibody for 48 hours. After this time, the levels of TLR9 (A) mRNA were determined using PCR in 1) splenocyte, 2) activated and 3) naïve CD4 T cells and 4) water control. (B) TLR9 protein levels in naïve (shaded) and activated (bold line) CD4 T cells were determined using flow cytometry. CD4 T cells were also stained with isotype antibody (light line).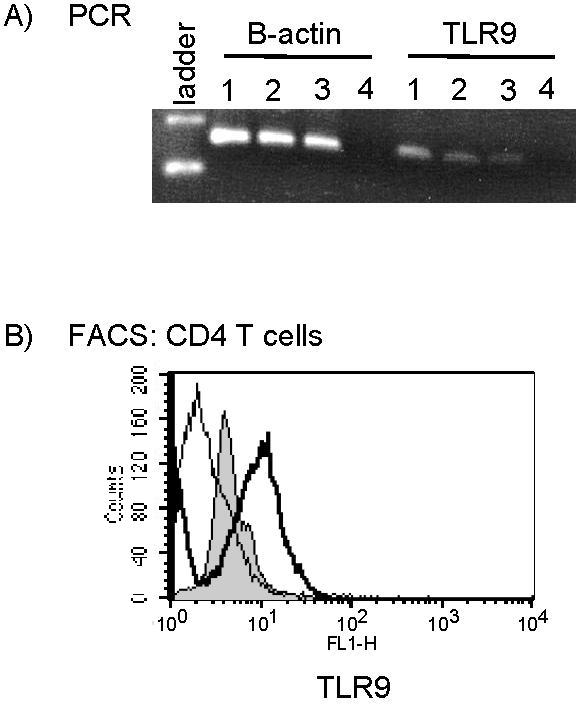
- Figure S2. Bcl-2 expression levels decrease in TLR9–stimulated, irradiated T cells following inhibition of Chk1, Chk2 or ATM (JPG, 57.3 KB) -
CD4 T lymphocytes were treated as described in Figure 1. Prior to IR exposure, UCN-01 or DBH which inhibit Chk1 and Chk2 activation, or wortmannin which inactivates PI3K activity, were added to cell cultures. The levels of anti-apoptotic protein bcl-xl and bcl-2 were determined by Western blot analysis 24 hours after IR (300 cGy).
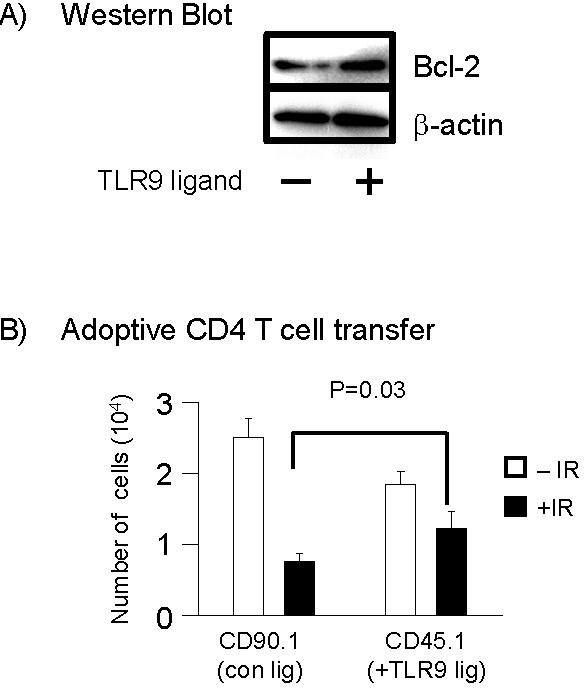
- Figure S3. In vitro-activated,TLR9-stimulated CD4 T cells show preferential survival over non-TLR9-stimulated cells in vivo and show enhanced Bcl-2 expression (JPG, 57.5 KB) -
CD45.1 and CD90.1 CD4 T-cells were purified by negative selection and followed by positive selection using magnetic beads. CD4 T cells were activated with plate-bound anti-CD3 and CD28 antibody for 48 hours. After this time, CD45.1 CD4 T cells were cultured in fresh media in the presence of TLR9 ligand (10 µg/ml CpG-ODN) for 24 hours while CD90.1 CD4 T cells were cultured in fresh media in the absence of TLR9 ligand. After incubation with TLR9 ligand, the levels of bcl-xL,bcl-2 and -actin were determined prior to adopitve transfer into mice. (B) T cells were mixed at a 1:1 ratio and intravenously injected into congenic CD90.2 CD45.2 BL/6 mice (n=3). Twenty four hours after adaptive transfer mice underwent TBI (300 cGy). Ten days after TBI, the numbers of CD45.1 and CD90.1 cells were determined by flow cytometry by staining spleen cell suspensions with antibodies to CD4, CD45.1, or CD90.1. Data shown are the means ± s.d.; The P values were determined by use of the student T-test. The p-values between IR and non-IR cells were also statistically signicant while the numbers of non-IR cells between CD90.1 and CD45.1 not significant (P> 0.1).
-actin were determined prior to adopitve transfer into mice. (B) T cells were mixed at a 1:1 ratio and intravenously injected into congenic CD90.2 CD45.2 BL/6 mice (n=3). Twenty four hours after adaptive transfer mice underwent TBI (300 cGy). Ten days after TBI, the numbers of CD45.1 and CD90.1 cells were determined by flow cytometry by staining spleen cell suspensions with antibodies to CD4, CD45.1, or CD90.1. Data shown are the means ± s.d.; The P values were determined by use of the student T-test. The p-values between IR and non-IR cells were also statistically signicant while the numbers of non-IR cells between CD90.1 and CD45.1 not significant (P> 0.1).