Robinson et al. 10.1073/pnas.0800742105. |

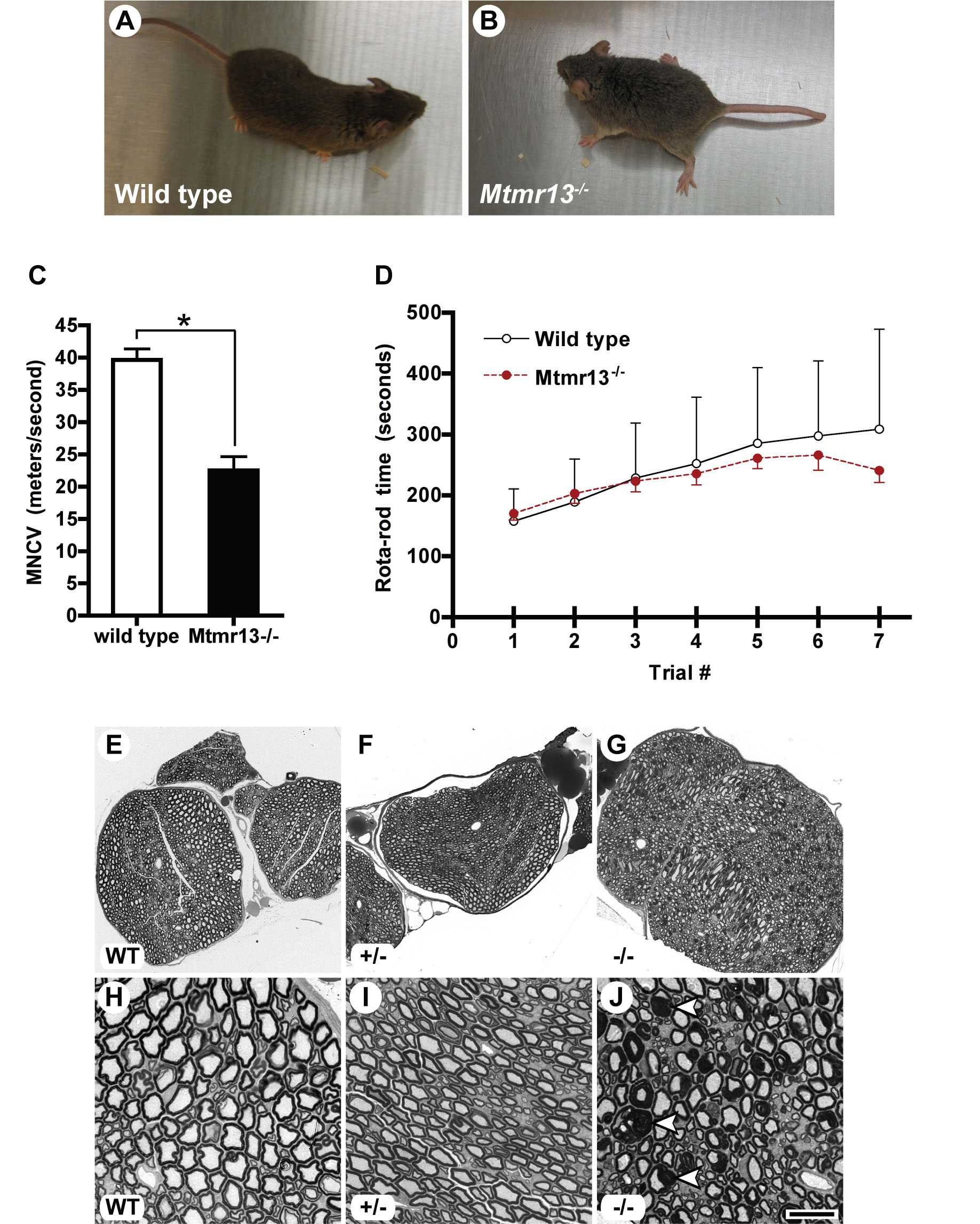
Fig. 5. Phenotypic evaluation of Mtmr13-deficient mice. (A and B) Abnormal stance in Mtmr13-deficient animals. Some Mtmr13-deficient mice transiently show wide-placed hind paws after placement on a flat surface. Six-month-old male mice are shown. (C) Motor nerve conduction velocity (MNCV) was evaluated in wild-type and Mtmr13-/- mice at 8 months (n = 6 for wild type and 7 for Mtmr13-/-, respectively). MNCVs were analyzed in triplicate for each animal and are presented as the mean ± SEM (*, P < 0.0001). (D) Neuromuscular (RotaRod) evaluation of wild-type and Mtmr13-/- mice. Male mice, aged 6 months, were put through a 4-day RotaRod testing protocol (see Materials and Methods). Each trial represents three runs of the RotaRod per animal (n = 7 for each genotype). Average times on the RotaRod are presented on the y axis ± SEM. Significant differences in RotaRod performance between wild-type and Mtmr13-/- animals were not observed. (E-J) Abnormal myelin morphology in Mtmr13-deficient peripheral nerves. Sciatic nerves were analyzed at 7 months as described in Materials and Methods. Nerves from Mtmr13-/- mice (G and J) contain numerous fibers with extensive redundant loops of myelin (arrowheads in J), which are not observed in wild-type (E and H) or heterozygous (F and I) mice. [Scale bar: 100 mm (E-G) and 20 mm (H-J).]
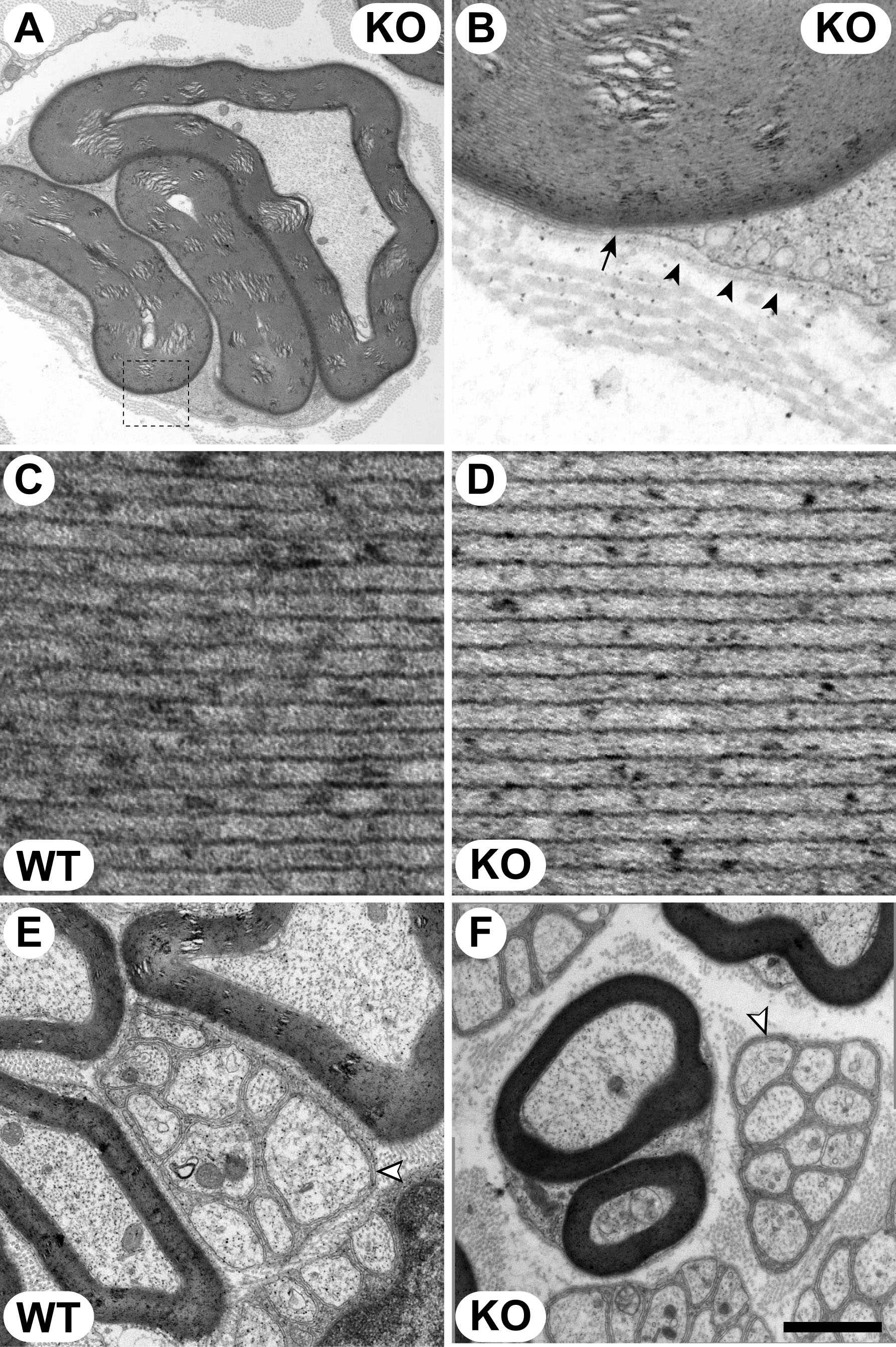
Fig. 6. Ultrastructural analysis of Mtmr13-/- Schwann cells in peripheral nerves. Sciatic nerves from wild-type (C and E) and Mtmr13-/- (A, B, D, and F) mice were analyzed at 7 months. Ultrathin (70-nm) transverse sections were analyzed by electron microscopy as described in Materials and Methods. (A and B) Myelin outfolds in Mtmr13-/- Schwann cells are bounded by the Schwann cell plasma membrane (arrow) and a morphologically normal basal lamina (arrowheads). B is a magnification of the indicated portion of A. (C and D) Myelin periodicity and compaction are normal in Mtmr13-/- nerves. (E and F) Nonmyelinating Schwann cells (white arrowheads) in Mtmr13-/- nerves (F) appear morphologically similar to those in wild-type nerves (E), with nonmyelinated axons segregated from each other and enclosed by interdigitating Schwann cell processes. [Scale bar: 2.2 mm (A), 0.32 mm (B), 44 nm (C and D), 1 mm (E), and 1.75 mm (F).]

Fig. 7. Peripheral nerve pathology in Mtmr13-deficient mice has an early onset and worsens with age. Mice were analyzed at postnatal days 14 and 28 (P14 and P28) and 2, 4, 7, and 14 months (mo). Semithin (0.5 mm) or ultrathin (70 nm) transverse sections of mid-sciatic nerves were examined by light microscopy or EM. (A) Myelin infoldings and outfoldings (arrowheads in Mtmr13-/- images) are detected in P14 Mtmr13-/- mice and increase in frequency with age. (B) Quantification of progression of dysmyelination in Mtmr13-/- mice. Either two or three Mtmr13-/- animals were analyzed at each time point. The percentage of abnormal fibers (fibers with one or more myelin outfolds or infolds) is presented as mean ± SEM. *, for the 1- and 2-month means, the SEMs were 0.34 and 0.43, respectively. (Scale bar: 20 mm.)
SI Materials and Methods
Disruption of Mtmr13.
We have assumed that the cDNA sequence corresponding to GenBank accession no. XM_133816.2 represents the authentic mouse Mtmr13 mRNA. XM_133816.2 is a 7,088-bp sequence containing an ORF of 5,544 bp (1,847 aa; GenBank accession no. XP_133816.2). All cDNA/mRNA sequence numbers provided here are based on the assumption that the adenosine of the initiator methionine of XM_133816.2 is nucleotide 1. We used the XM_133816.2 sequence as query in a BLAST search of the mouse genome. This search indicated that the Mtmr13 gene is composed of 40 exons strung over ~300 kb on chromosome 7 (region F2). Mtmr13 is flanked by the genes for adrenomedullin and SWAP-70, which are, respectively, 13 and 24 kb (5' and 3') of the gene.BayGenomics ES cell line RRF511 contains an exon-trapping plasmid (pGT0lxf) integrated within intron 16-17 of the Mtmr13 gene. Gene trapping with pGT0lxf is described at the Sanger Institute web site (www.sanger.ac.uk/PostGenomics/genetrap). The mRNA resulting from the disrupted gene is predicted to contain exons 1-16 followed by an in-frame bgeo ORF, a stop codon, and a poly(A) signal. The resulting protein is predicted to consist of Mtmr13 residues 1-620 fused to the bgeo protein.
All animal work was approved by and conformed to the standards of the University of California at San Diego Institutional Animal Care and Use Committee. Mtmr13+/- ES cells (strain 129/Ola) were injected into C57BL/6 blastocysts, and chimeric mice were obtained. Male chimeras were bred to C57BL/6 females, and germ-line transmission of the mutated allele was confirmed by PCR-based genotyping by using primers specific for the bgeo sequence [primers 400 (TTATCGATGAGCGTGGTGGTTATGC) and 401 (GCGCGTACATCGGGCAAATAATATC)]. Genomic (g) DNA was isolated from mouse tails using the DNeasy Blood and Tissue Kit (Qiagen). Genomic PCR was by standard methods and generally involved 40 cycles of amplification. The genomic site of pGT0lxf integration was identified by using PCR to scan through intron 16-17 with various 5' primers of intronic sequence and a 3' primer specific for pGT0lxf (primer 541; AGGCCCCTTCGCTAGAAGCG). Mtmr13 genotyping was performed by using primers 599 (GGGTTCTCTATGTGGCCTGACC), 600 (CTCCAGTCTCCTCCACCCTGG), and 601 (GGATTTGTGCTGGAATCTGGATACC) in a three-primer PCR.
RNA Analysis.
RNA was isolated from mouse brains by using TRIzol reagent (Invitrogen). RT-PCR (30-35 cycles) was by standard methods and used primers 368 (GACAGTATCGGCCTCAGGAAGATCG), 369 (GTAGTGCCAGCAGGTCCACCC), and 372 (GGTGGTCCTGTACACAGGTGTAGGC). To confirm disruption of the Mtmr13 mRNA, we isolated brain RNA from RRF511 heterozygous mice and performed RT-PCR using oligonucleotides 369 (exon 14) and 368 (bGeo). An RT-PCR product of the expected size (531 bp) was amplified from the brains of RRF511 heterozygous, but not wild-type, mice. The mutant Mtmr13 RT-PCR product was sequenced to confirm that exon 16 is fused to the bgeo sequence as predicted. In the mutant fusion protein, glutamine (Q) 620 is the final Mtmr13 amino acid; Q620 is followed by valine-10 of the bgeo protein sequence. Thus, the sequence of the fusion protein junction is Mtmr13-IIRMMNCTLQ620-V10PGPENQRRR-bgeo. The resulting mutant fusion protein is predicted to be 1,942 aa in length and to have a molecular mass of 218,945 Da.RT-PCR with primers 369 (exon 14) and 372 (exon 18) yielded an unexpected 338-bp band when RNA from RRF511 heterozygous or homozygous mice was used. Sequencing of this RT-PCR product established that the band results from splicing of exon 16 to exon 18 (Fig. 1C). We refer to this mRNA as DEx17. Because the DEx17 splicing maintains the ORF, the potential for the translation of a DEx17 Mtmr13 protein exists. Indeed a trace amount of an ~191 kDa protein is detected in Mtmr13-/- brain immunoprecipitates when immunoblots are highly overexposed. This protein may represent the DEx17 translation product. Densitometry of immunoblots estimated the abundance of the putative DEx17 protein in Mtmr13-/- brains to be ~2.5% of wild-type levels.
Protein Analysis.
Brain protein extracts were prepared by homogenizing fresh brains from 6-month-old animals in ice-cold lysis buffer (120 mM NaCl/50 mM Tris, pH 8.0/0.5% Triton X-100/100 mM NaF/1 mM phenylmethylsulfonyl fluoride/1 mg/ml leupeptin/10.5 mg/ml aprotinin/1 mg/ml pepstatin/1 mM benzamidine/1 mM sodium orthovanadate) and subsequently clearing the extract by centrifugation at 17,000 ´ g. Sciatic nerves (from sciatic notch to knee) were removed from killed animals and frozen in liquid nitrogen. Nerves from two 10-month-old animals were pooled and ground to a powder by using a nitrogen-chilled mortar and pestle. This material was suspended in lysis buffer, homogenized by using a Dounce homogenizer, and clarified by centrifugation as above. For SDS/PAGE and immunoblotting 26 mg and 5 mg of protein from brain and sciatic nerve, respectively, was resolved in 4-12% NuPAGE BisTris gels in Mops buffer (Invitrogen). Mtmr13 was immunoprecipitated from brain or sciatic nerve extracts as described previously (1), except that either 10 mg of extracted brain protein or 0.4 mg of sciatic nerve protein was used for immunoprecipitation (IP). Immunoprecipitates and extracts were resolved by SDS/PAGE, transferred to PVDF membranes, and analyzed by immunoblotting. Densitometry was performed as described previously (1). Antibodies used were rabbit anti-MTMR2 and anti-MTMR13 (1) and rabbit anti-MAG and mouse anti-b-tubulin (Santa Cruz Biotechnology).Nerve Conduction Velocity (NCV).
Wild-type and Mtmr13-/- mice (n = 6 and 7, respectively; age 8 months) were examined by using a previously described procedure (2). Briefly, mice were anesthetized with isoflurane (4% in O2 for induction, 2-3% for maintenance), and a nerve temperature of 37°C was maintained by using a heating lamp and a thermal pad linked to a temperature regulator and a rectal thermo-probe. Motor NCVs were measured in the sciatic nerve interosseus muscle system. Sciatic nerves were stimulated by using single, supramaximal square wave pulses of 4-8 V and 0.05-ms duration using two fine needle electrodes, which were placed at the Achilles tendon and sciatic notch. Evoked electromyograms were recorded from the interosseus muscles of the ipsilateral foot by using two fine needle electrodes and analyzed on a digital storage oscilloscope. Distances between sites of stimulation were measured by using calipers, and conduction velocities were calculated by dividing the distance between sites of stimulation (in millimeters) by the distal motor latency (in milliseconds) (2). Readings were taken in triplicate for each animal, and a median NCV value was calculated. NCV values for wild-type and Mtmr13-/- mice are expressed as an average value (meters/second) ± standard error of the mean. An unpaired t test was performed to evaluate statistical significance by using Prism 4 software.Ambulatory (RotaRod) Evaluation.
Male wild-type and Mtmr13-/- mice (n = 7 for both genotypes; age 6 months) were tested on an accelerating RotaRod (model 7650; TSE Systems). RotaRod speed was accelerated from 5 to 50 rpm over 5 min, and the time until a mouse fell off the rod was recorded. Each animal was subjected to two trials before being returned to its cage. Three replicates were performed in a day, and the entire procedure was repeated on three additional, nonconsecutive days. Presented data are the average time to a fall for each of the seven trials for wild-type and mutant mice. Data were plotted on XY graphs with standard deviation, and Student's t tests were used to evaluate statistical significance.Morphological Analysis.
Anesthetized mice were perfused with 4% paraformaldehyde/1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Nerves were removed, further fixed overnight in the same buffer, and prepared for EM by postfixation with OsO4 and en bloc staining with uranyl acetate. Ultrathin (70 nm) sections were cut and contrasted further with uranyl acetate and lead nitrate, and grids were viewed and photographed by using either a Philips CM-10 transmission electron microscope (FEI Company) fitted with a 794 Multiscan CCD camera (Gatan) or a JEOL 1200EX II transmission electron microscope fitted with an ORIUS SC600 digital camera (Gatan).For semithin morphological analysis, nerves were processed as described above except that 0.5 mm cross sections were cut and stained with toluidine blue. Mid-sciatic nerve sections were examined and photographed by using a Zeiss Axioplan 2 upright microscope. Nerves from P14 and P28 mice were evaluated by using EM. To determine the percentage of sciatic nerve fibers with myelin outfoldings/infoldings, two or three Mtmr13-/- mice of each age (14 days, 28 days, and 2, 4, 7, 12, or 14 months) were analyzed. For each mouse, three or four random fields were analyzed in sciatic nerve cross sections. An average of 466 fibers was counted per mouse. An abnormal fiber was defined as one containing one or more redundant loop(s) of myelin flanking a primary myelinated axon, or one containing one or more myelin infolds (into the axon). Mid-sciatic nerve sections from heterozygous mice were examined at 7 and 14 months and appeared indistinguishable from those of wild-type mice when examined by either light microscopy or EM.
1. Robinson FL, Dixon JE (2005) The phosphoinositide-3-phosphatase MTMR2 associates with MTMR13, a membrane-associated pseudophosphatase also mutated in type 4B Charcot-Marie-Tooth disease. J Biol Chem 280:31699-31707.
2. Garcia ML, et al. (2003) NF-M is an essential target for the myelin-directed "outside-in" signaling cascade that mediates radial axonal growth. J Cell Biol 163:1011-1020.