[View Larger Version of this Image]
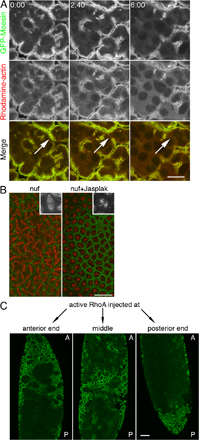
Figure S4. Suppression of nuf phenotypes. (A) Nuf is required for F-actin maintenance at the cellularizing furrows. Rhodamine-actin was injected into nuf embryos expressing GFP-Moesin at nuclear cycle 13 and furrow F-actin dynamics was followed during early cellularization. Both GFP-Moesin (green in merge) and injected Rhodamine-actin (red in merge) are not stably maintained at the cellularization furrow (arrows) in nuf embryos. (B) Loss of F-actin at the furrow in nuf embryos is suppressed by Jasp treatment. The furrow and microtubules were labeled with GFP-Moesin and Rhodamine-Tubulin, respectively, in nuf embryos. Embryos were injected with either 1 mM Jasp or DMSO at cycle-13 early prophase. At metaphase, extensive furrow breaks and spindle fusions were observed in DMSO-injected nuf embryo, whereas the furrow defects were greatly reduced in Jasp-injected nuf embryo. Jasp also affected spindle morphology (inset to show higher magnification images). Insets = 2×. (C) Loss of F-actin at the furrow in nuf embryos is suppressed by constitutively active RhoA injections. Furrows were labeled with GFP-Moesin. A, anterior; P, posterior. Constitutively active RhoA(Q63L) was injected into early cellularizing nuf embryos either anteriorly (n = 5), posteriorly (n = 5), or in the middle (n = 5). After ∼30 min after injection, furrows persisted only in the regions close to RhoA(Q63L) injections and were largely depleted from the rest of the embryo. Bars: (A) 10 µm; (B and C) 20 µm.