Blood, Vol. 112, Issue 9, 3601-3614, November 1, 2008
Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells
Blood Zambidis et al. 112: 3601
Supplemental materials for: Zambidis et al
Files in this Data Supplement:
- Document 1. Supplemental materials and methods (PDF, 73.3 KB)
- Table S1. Human-specific primers used for real-time qRT-PCR (PDF, 82.6 KB)
- Table S2. Gene Microarray list of hEB differentiating in SF/BVF2H conditions (Day 0 = purified undifferentiated H1 hESC) (XLS, 9.35 MB)
- Figure S1. Mesodermal morphogens and hematopoietic growth factors potentiate highly efficient production of YS-like primitive and definitive hematopoietic progenitors from hEB in SF medium (JPG, 599 KB) -
(A) Optimized SF hematopoietic differentiation strategy: 50 ng/ml BMP4 (B), 50 ng/ml VEGF (V), 50 ng/ml FGF1 + 5 µg/ml heparin (FGF1), and 50 ng/ml FGF2 + 5 µg/ml heparin (FGF2), or no factor (0), were added to non-adherent SF LDM hEB suspension cultures starting on day 2, with hemi-depleting medium + GF changes until day 9 (Stage I). On day 9, hEB were harvested, enzymatically-disaggregated, and re-cultured onto gelatinized tissue culture plates in SF LDM medium supplemented with a broad cocktail of inductive/expansive hematopoietic growth factors (Stage II; GF=BMP4, SCF, Flt3 ligand, TPO, G-CSF, GM-CSF, IL-3, IL-6, EPO, VEGF), with or without FGF1/heparin (FGF1), or FGF2/heparin (FGF2). Day 15 hEB (Stage II) were disaggregated into single cell suspensions, passed through a 70 µm strainer, and evaluated for CFC potential in H4436 SF methylcellulose with hematopoietic growth factors, as described in Methods. Primitive, definitive, and mixed multipotent colony morphologies arising from variously-treated hEB cells were scored, as described in Methods (MHE, MIXED-P, MIXED-D, BFU-e-P, EryP, CFU-e-d, BFU-e-d, monocyte/macrophage CFU). Shown is a representative of three independent experiments +/− SEM of duplicate CFC cultures for each condition. We noted that adding the broader GF cocktail to differentiating hEB earlier than day 8–9 of hEB differentiation only marginally improved the CFC potential of hEB (data not shown), compared to the minimal BVF2H combination alone. Inclusion of BMP4 and VEGF, both previously demonstrated to be necessary for hematopoietic differentiation of hESC,5–7 were similarly critical in our differentiation system (data not shown), Further inclusion of EPO along with BMP4 and VEGF in our SF GF cocktail after day 9 hEB formation (Stage II) was also especially potent, with augmentations of both primitive and definitive CFC at days 14–15, including erythroid and multi-potent, mixed colonies, by an additional 5–10× fold (data not shown). Interestingly, the inclusion of FGF2/heparin (but not FGF1/heparin) to various GF cocktails during days 4–15 of hEB differentiation dramatically augmented (7–12− fold) the numbers of both primitive and definitive YS-like hematopoietic cell colonies derived from day 15 hEB (A). Similarly potent effects of FGF2 were reported by another group, using a slightly different SF hEB differentiation protocol.8 All types of colonies were greatly expanded with inclusion of FGF2, including primitive erythroid, definitive erythroid, primitive myeloid, and mixed primitive/definitive colonies, thus suggesting an expansive effect on either early multipotent, or lineage-committed progenitors. This dramatic FGF2 effect was likely due to synergism with VEGF and BMP4 in our GF mixture, since there was minimal effect on hEB CFC output when combinations of FGF2/heparin, or VEGF/BMP4 were added alone during days 4–15 differentiation (A and additional data not shown). (B) Phenotypes of YS-like primitive and definitive hematopoietic colonies generated in SF cultures. hEB were differentiated in SF media supplemented with BVF2H, and single-cell suspensions from day 9 or day 14 hEB were re-cultured for CFC potential, as described in the Text. Erythroid (A) or mixed, erythro-myeloid (B) colonies were scored morphologically as primitive (BFU-e-P, EryP, MIXED PRIM), or definitive (BFU-e-D, CFU-e-D, MIXED DEF). CFC assays from plated CD34+-enriched neonatal CB cells were conducted in parallel, as a positive control, to generate adult-type definitive colonies (CD34+ CB DEFINITIVE, BFU-e-D, CFU-e-D, GM-CFC, GEMM-CFC). Erythroid colonies with “brilliant red” hemoglobinization (BFU-e-P, EryP; Day 9 hEB PRIMITIVE), and containing large nucleated cells were generated from day 7 to 12 day hEBs. Erythroid colonies containing a brownish, “salmon-red” hemoglobinization were generated from day 12 to 20 day hEBs (BFU-e-D, CFU-e-D: day 14 hEB DEFINITIVE), and were morphologically similar to definitive erythroid CFU obtained from CD34+ cord blood cells (CD34+ CB DEFINITIVE). ~Ten colonies of each designated morphology were plucked from methylcellulose cultures and pooled for globin, or surface marker expression by FACS analysis. Pooled colonies were analyzed for expression of intra-cytoplasmic adult (HbA), fetal (HbF), embryonic epsilon hemoglobin (Hbε) chains, or membrane expressions of erythroid-specific markers CD71, glycophorin A (CD235a), CD36, and embryonic pan-hematopoietic marker CD43.40 Although all types of erythroid CFCs had similar surface expression of CD71/CD235a (glycophorin A), and expressed abundant amounts of fetal hemoglobin (HbF), only day 14 to 20 BFU-e-D and CFU-e-D expressed adult hemoglobin A (HbA). Detection of co-expression of both HbF and HbA in BFU-e-D from CD34+ CB progenitors was used as a positive control. (C) Distinct MHE clusters (left panel) which consist of adherent mesenchymal and endothelial stromal cells, and bud off multipotent hematopoietic blasts,24 are generated from day 4–9 hEB cells. Mixed-definitive CFU from day 15 hEB (MIXED DEF) contain macrophages and erythroid cells with adult hemoglobin (HbA) expression (B, right panel) in comparison to Mixed-primitive erythroid-macrophage CFU from day 9 hEB (MIXED PRIM) which express only embryonic epsilon and fetal globins (B, right panel). In contrast, multipotent GEMM CFU from CD34+ CB (CB GEMM) express little epsilon, and abundant beta globin than both MIXED PRIM and MIXED DEF CFU. (D) Growth factor requirements of blast colonies generated in SF conditions. Single-cell suspensions from day 5–10 hEB were re-suspended in SF methylcellulose medium in the presence of no growth factor (no GF), or combinations of hemangioblastic growth factors, including 50 ng/ml SCF (S), 50 ng/ml BMP4 (B), 50 ng/ml VEGF (V), 50 ng/ml FGF2 + 5 µg/ml heparin (FH), 50 ng/ml TPO (T), or 50 ng/ml IL-6. Shown are representative results of experiments done >3 times in duplicates for each condition, from day 9 hEB cells.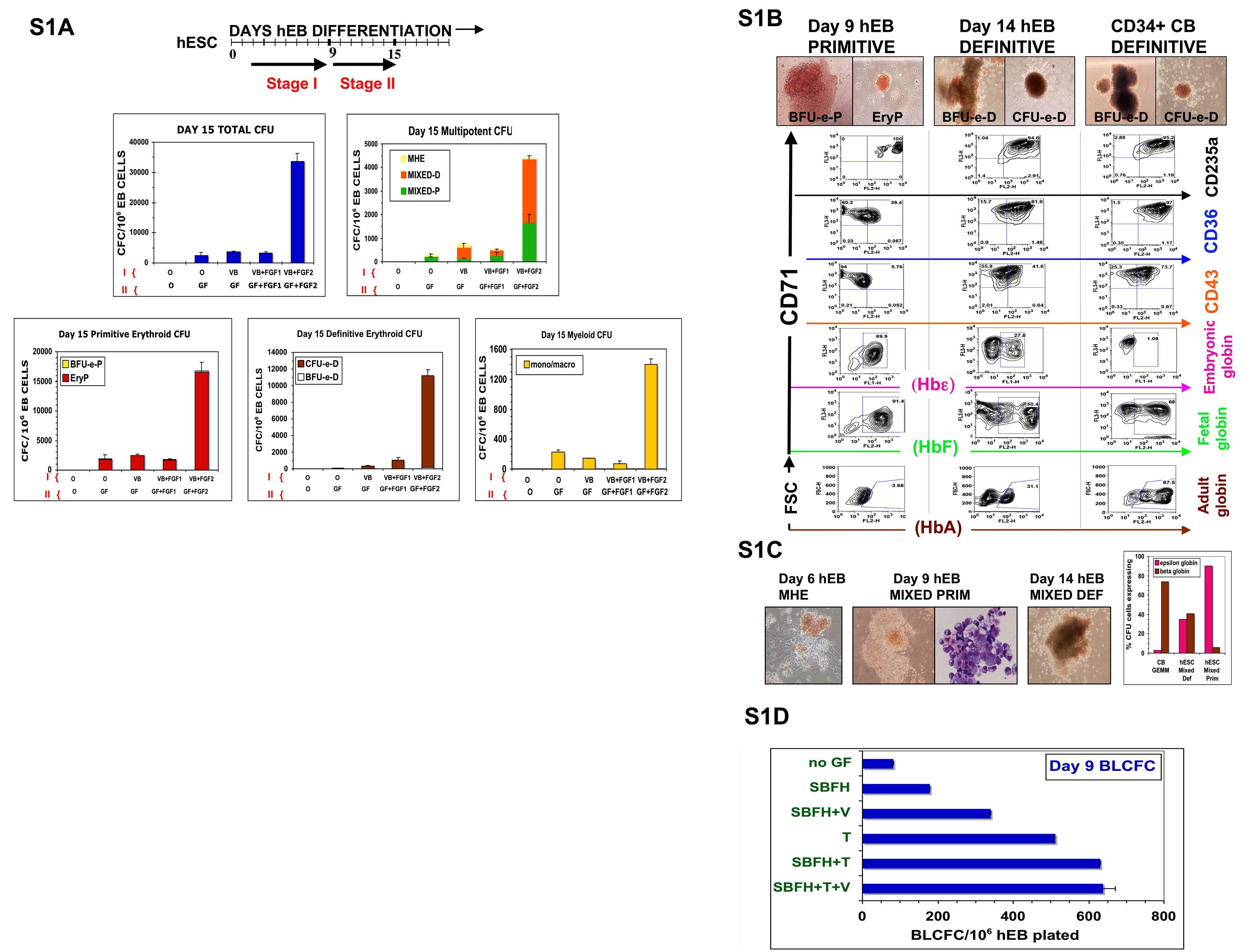
- Figure S2. Co-expression of surface ACE/BB9 with other hemato-endothelial markers on hEB cells and hemangioblasts (JPG, 691 KB) -
(A–E) hESC (H1; WA01) were differentiated into hEB in suspension cultures prior to FACS analysis in SF medium in the presence of BMP4, VEGF, and FGF2/heparin alone (A–E, days 5–9 hEB; S2D, S2E, days 9–11 hEB timepoints). hEB were further cultured from days 9–14 (D, E, day 14 hEB+GF) as adherent hEB on gelatinized plates in the presence of a broader hematopoietic growth factor cocktail, as above (hEB+GF; BMP4/Flt3L/TPO/VEGF/SCF/G-CSF/GM-CSF/IL-3/IL-6/FGF2/EPO) prior to FACS analysis and enzymatic disaggregation. Surface expressions of hEB cells for KDR/flk-1, CD34, CD31 (general hemato-endothelial markers), CD43 (embryonic pan-hematopoietic marker), and CD45 (definitive pan-hematopoietic marker), and correlation to co-expression of ACE/BB9 on these hEB cells was evaluated by FACS. (A, B) A large proportion (~10–35%) of hEB cells express KDR/flk-1 during days 5–9 of hEB differentiation. (C) Hematopoietic potential of purified ACE+KDR+ hEB populations. Day 5 hEB were disaggregated into single-cell suspensions and FACS-purified into three sorted populations: KDR+BB9− (SORTED GATE A), KDR+BB9+ plus KDR−BB9+ (SORTED GATE B), and KDR−BB9− (SORTED GATE C). FACS-purified day 5 hEB cells were enumerated by post-sort FACS analysis, and 5,000–10,000 single, viable cells were plated in duplicate into hemangioblastic CFC assay conditions, as above, onto fibronectin-coated dishes. (S2C, lower panel) Mixed, fibronectin-binding hemato-endothelial (HE), or hematopoietic-only (H) colonies arising from single, purified cells were scored 10–14 days later, as above. Only the population expressing BB9/ACE (which contained cells co-expressing dim surface KDR/flk-1, as well as a proportion expressed no KDR/flk-1; C, SORTED GATE B) demonstrated significant hemato-endothelial, or hematopoietic-only potential. KDR+(high)BB9− populations (SORTED GATE A) expanded only adherent cells, with end-differentiated endothelial morphologies (data not shown, and similar to cells in Figure S3B below).
- Figure S3. Characterization and comparison of adherent endothelial cells derived from either MHE clusters, or replated day 5 hEB blast colonies (JGP, 740 KB) -
Hemato-endothelial cells derived from a MHE cluster (A; 100× mag.), or from a secondary hemangioblastic colony derived from single, replated day 5 blast colony (B; 100× mag.), which were growing in methylcellulose cultures were washed of non-adherent cells, and incubated overnight in 10 µg/ml Dil-conjugated acetylated LDL (Dil-AcLDL) in SF LDM. Twenty-four hours later, adherent cells were fixed, permeabilized and further immunostained with Fluorochrome-conjugated antibodies for mesenchymal cell-specific vimentin (FITC-conjugated), CD31 (FITC-conjugated), or endothelial cell-specific Ulex Europeaus agglutinin (FITC-conjugated). Nucleii were counterstained with DAPI. Note that MHE clusters, unlike adherent cells derived from blast colonies, contained not only (robust) Dil acetylated-LDL+ cells, but also many cells that did not take up acetylated LDL, but that stained strongly for mesenchymal-specific vimentin antigen.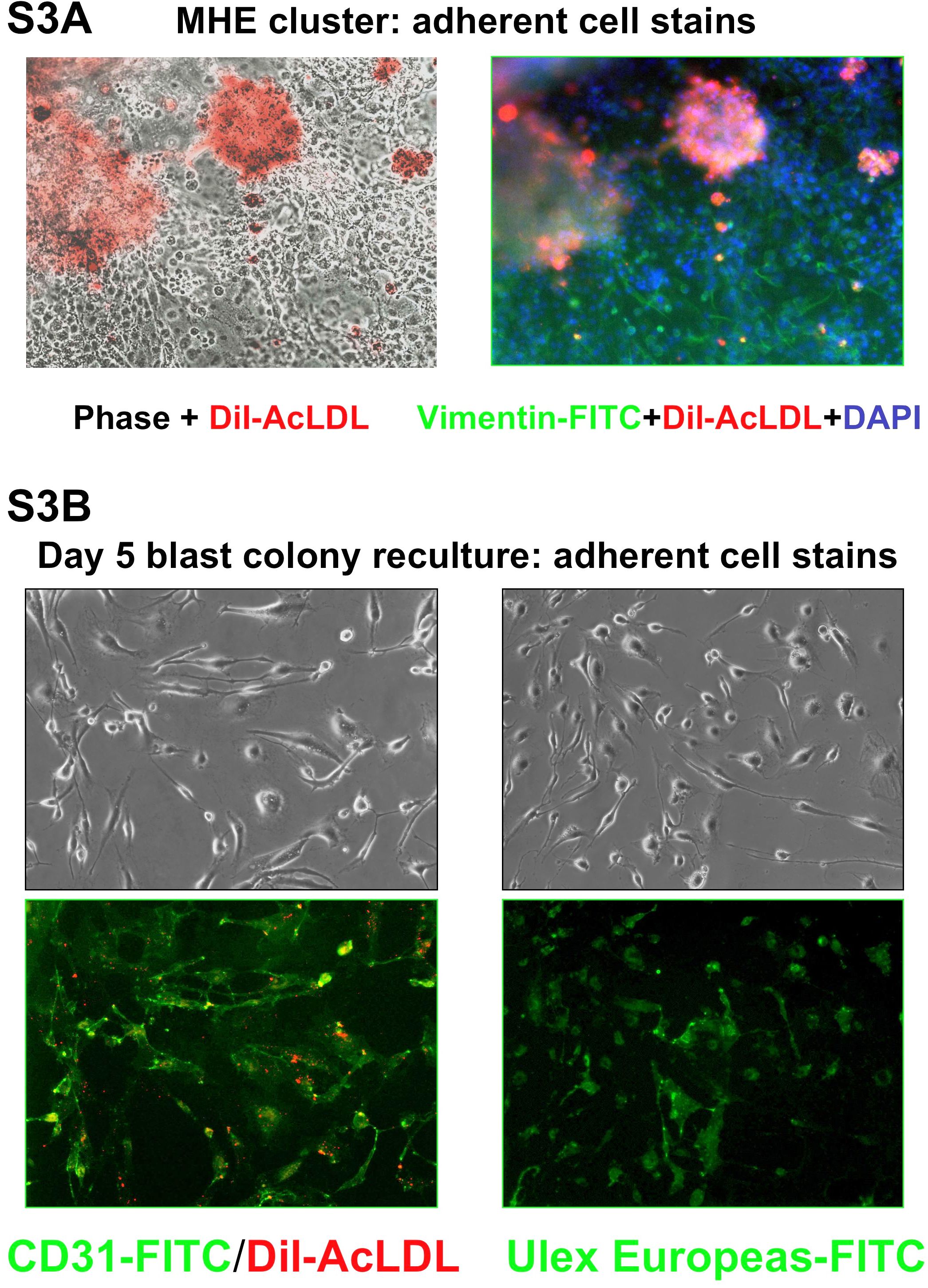
- Figure S4. Surface marker and molecular expression analyses of FACS-purified BB9/ACE+ hEB cells (JPG, 711 KB) -
(A) Day 9 hEB were enzymatically-disaggregated into single cell suspensions and purified into ACE/BB9+ and BB9− fractions by FACS sorting with mAb BB9-APC, as in Fig. 3B. Sorted cells were further costained with PE-conjugated antibodies for various hemato-endothelial progenitor markers including CD34, CD31, KDR/flk-1 CD164, CD2019, CD117, CD43, CD41a, and CD45. (B) RNA was harvested from FACS-purified day 9 hEB cells and transcripts for the Actin (act), CDX4, CDX2, Erythropoietin receptor (EpoR), FLT3 receptor, MLL, HOXB4, LMO2, AML1a and AML1b isoforms (AML1a/b), AML1c, C-MYB, SCL, GATA1, PU.1, and thrombopoietin receptor (MPL) were assayed by qRT-PCR, as described in Methods. Shown are relative actin-normalized quantitative comparisons, using the 2−ΔΔT method. Fold change expression differences indicate levels of transcripts of sorted BB9+ hEB cells in comparison to sorted BB9− hEB cells (B, top panel). Shown in (B), middle panel, are actin-normalized comparisons of fold change expression of blast colonies to BB9+ hEB cells, of indicated transcripts. Bottom panel shows agarose gels from BB9+, BB9− and pooled blast colonies (Blasts) of PCR products at linear amplification for assayed transcripts. These studies demonstrated that BB9+ hEB cells, which gave rise to all multipotent hematopoietic progenitors, were devoid of significant expression of mature hematopoietic markers (e.g. CD45, CD43, CD41), and yet co-expressed multiple markers associated with hemato-endothelial progenitor activity (e.g. CD34, CD31, KDR, CD164, CD201, CD117; c-kit receptor). The increased expression of these multiple transcripts in blasts is consistent with the known molecular phenotypes of expressed regulatory factors in embryonic hematopoietic progenitors.10
- Figure S5. Global gene expression analysis of undifferentiated hESC and, hEB cells differentiated with SF BVF2H conditions (JPG, 543 KB) -
(A) Gene Tree Clustering of hEB differentiation spectrum showing global gene expression patterns during BVF2H differentiation. Analysis was done using changes relative to day 10 hEB with higher or lower than 16-fold changes with Pearson correlations for similarity measure and average linkage clustering algorithms. Top fifty up- or down-regulated gene clustering was done with additional filtering to choose probes with RefSeq ID only, and with significant changes at either day 5 or day 10 of hEB differentiation. (B) Relative expression changes during hEB differentiation for pluripotency-associated genes (SOX2, OCT4/POU5F1, NANOG), lineage-associated (AFP, SOX17, GATA4(endoderm), PAX6 (ectoderm)), and renin-angiotensin system (RAS) genes (renin, AGTR1, AGTR2, angiotensinogen (AGTN). These array results were validated on unsorted and purified BB9+ hEB using qRT-PCR methods and primers as described in the text and in Table S1 (data not shown). Full normalized gene array results are included in Table S2.