Blood, Vol. 113, Issue 4, 929-935, January 22, 2009
N-Glycans of ADAMTS13 modulate its secretion and von Willebrand factor cleaving activity
Blood Zhou and Tsai 113: 929
Supplemental materials for: Zhou and Tsai
Files in this Data Supplement:
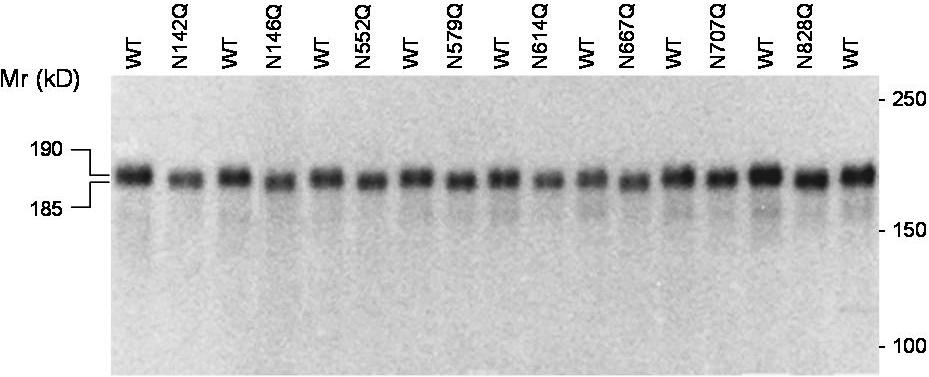
- Figure S1. N-glycan site mutation decreases the molecular weight of ADAMTS13 (JPG, 41.7 KB) -
Each of the 8 potential N-glycosylation sites in the metalloprotease, spacer-TSR #2, and TSR #4 domains were replaced with glutamine (Q). The wild type or mutant proteins in HEK 293T cell secretion were analyzed by SDS PAGE under reducing conditions to detect difference in the molecular weight. The proteins were visualized by immunoblotting with anti-V5. This study was not intended for comparison of protein concentration as analysis under reducing conditions increased the variability of the protein level on the blot.