Blood, Vol. 113, Issue 9, 2108-2117, February 26, 2009
Sphingosine kinase regulates the rate of endothelial progenitor cell differentiation
Blood Bonder et al. 113: 2108
Supplemental materials for: Bonder et al
Mouse progenitor cell isolation, culture and colony forming assay
BM cells at 1 × 104 were plated in 0.3% agar culture medium containing GM-CSF (100 ng/ml, Prospec, Rehovot, Israel), G-CSF (10 ng/ml, Amgen, Thousand Oaks, CA), IL-3 (100 ng/ml, Prospec), SCF (100 ng/ml, IMVS, Adelaide, SA, Aust.), IL-6 (50 ng/ml, Prospec) or erythropoietin (EPO, 4 U/ml, Janssen-Cilag) in 35mm culture dishes (Falcon, NY, USA) or at 1.5 × 105 cells/cm2 cultured on fibronectin (Fn; 50 µg/ml; Roche) coated 6-well trays in either 20% fetal calf serum (FCS; JRH, Brooklyn, Vic, Australia) or charcoal stripped 20% FCS M199-medium (JRH), prepared as outlined previously.64;65 Unless otherwise stated, all medium was supplemented with endothelial cell growth supplement and heparin (both at 50 µg/ml; BD Biosciences).
Animals
All mice used were of C57Bl/6 background with SK-1-KO mice previously described 44 and SK-1-Tg mice generated by Ozgene (Bentley, WA, Australia), where the pronuclear injection of human SK-1 cDNA was injected as a linear product flanked by the Tie2 promoter and enhancer (adapted from 66). The change in SK-1 activity in SK-1-KO and SK-1-Tg mice was confirmed in BM cells using the SK-1 enzymatic activity assay with SK-1-Tg mice exhibiting a 70–100% increase when compared to WT mice and SK-1-KO levels barely detectable (data not shown). All mice were housed under pathogen-free conditions at the IMVS and used between 6–8 weeks of age. All experimental procedures were approved by the Animal Ethics Committee of the IMVS and conform to the guidelines established by the “Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.”
Antigen Expression
mFlk-1 (BD Biosciences) expression was examined on Lin−/c-kit+ BM derived cells and Lin− blood cells, VE-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA) and CD34 (BD Biosciences) were assessed as per manufacturers’ instructions with appropriate isotype controls and flow cytometric analysis (Beckman Coulter XL-MCL, Gladesville, NSW, Australia). Further immunophenotyping was executed on day 2 and 7 mouse using FACS buffer (0.5% BSA, 2mM EDTA PBS, pH 7.2) where cells were stained with MAb directed against mouse CD146, CD45, c-Kit and prominin-1 (mouse homolog to human CD133) (Miltenyi Biotech); against mouse CXCR-2 and CXCR-4 (R&D Systems, Minneapolis, MN); against mouse CD34, Sca-1, CD14, CD11b, CD90, CD105, CD29 and CD49 (Biolegend, San Diego, CA); against mouse VE-Cadherin (eBioscience, San Diego, CA); against mouse CD31 and KDR (BD Pharmingen, San Diego, CA). Corresponding isotype controls were used. Stained cells were analyzed using FACSCanto (Becton Dickinson, San Diego, CA) and FlowJo software.
To detect von Willebrand Factor (vWF) and endothelial nitric oxide synthase (eNOS), cells were fixed in 4% paraformaldehyde (Sigma, St. Louis, MO) prior to permeabilisation with 0.1% Triton-X 100/PBS (Sigma) and blocking with 2% BSA (Sigma). Antibodies to vWF (Abcam, Cambridge, UK), or eNOS/NOS type III (BD Biosciences). Alexa secondary antibodies were used (Invitrogen) prior to counterstaining with DAPI-methanol (50 µg/ml, Sigma) and visualization using a Nikon Ti-U fluorescent microscope (Nikon, Chiyoda-ku, Tokyo) and analysis using NIS-Elements software.
Q-RT-PCR Analysis
For Q-RT-PCR genomic DNA was removed using a DNA-free kit (Ambion, Thebarton, SA, Aust.), 1 µg of total RNA or similar amount of mRNA extracted from the same numbers of cells were converted to first-strand cDNA using the SuperscriptIII reverse transcriptase (Invitrogen,). Primers were designed using Primer Express 1.5 program and purchased from GeneWorks (Hindmarsh, SA, Australia) as presented in Table S1. QuantiTect SYBR Green (QIAGEN) was used for Q-PCR reaction. Amplification parameters were 95°C for 10 minutes and cycling at 95°C for 10 s, 57–60°C for 20 s, and 72°C for 30 s for a maximum of 45 cycles using the RoboCycler thermal cycler (Corbett Research, Mortlake, NSW, Australia). Experion Automated electrophoresis station (BioRad, Hercules, CA) and Rotor-Gene Analysis Software version 6 was used to qualitate and quantitate the RNA.
Adenovirus Infection
For infection with adenoviral constructs, cells were exposed to one plaque forming unit/cell for 2 h in M119 medium with 2% FCS and a further 22 h with medium containing 20% FCS. Cells were infected with a dose of virus previously determined to lead to at least 6-fold increase in SK-1 activity in both complete and S1P depleted media (data not shown). The same dose of control EV adenovirus was used.
MTS Assay
Briefly, 1 × 104 BM cells were seeded into Fn-coated 96 well plates and on each day 30 µl of MTS solution was added and the cells were incubated for 4 h. The absorbance at 490 nm was measured using a multiwell plate reader (EL808, Bio-Tek Instruments, Winooski, VT), with wells containing medium, but with no cells serving as background.
Attachment and Migration Assays
Following extensive washing, adherent cells were counted using microscopy (IX70, Olympus Australia Pty Ltd, Mt Waverley, Vic, Aust.) and AnalySIS LS software (Olympus Soft Imaging Solutions GMBH, Planegg, Germany). Migration assays were performed using 8-µm Transwell plates (Corning Costar, Cambridge, MA), either uncoated or coated, with VEGF (100 ng/ml, Sigma) or 1% BSA as a binding control. Following trypsinization, 5 × 104 cells were seeded into the top chamber of the Transwell. 1% fetal calf serum ± soluble VEGF was added to the bottom well, to serve as a chemoattractant, and the plates were incubated at 37 °C for 3 h. Cells that migrated and adhered to the bottom surface of the Transwell membrane were fixed, stained (0.1% Crystal Violet), lysed with acetic acid and the absorbance read at 595 nm was measured using a multiwell plate reader (EL808, Bio-Tek Instrument).
SK inhibition Studies
SK inhibition and rescue studies used SKi (5 µM, Calbiochem, San Diego, CA.), S1P (1 µM, Cayman Chemical Co., Ann Arbor, MI, USA), pertussis toxin (PTX, 50 ng/ml, Sigma), JTE-013 (1 µM, Sapphire Biosciences, Redfern, NSW, Aust.), VPC23019 (10 µM, Avanti Polar Lipids Inc., Alabaster, AL) and FTY720 (100 nM, Cayman Chemical Co.) were administered at the initial seeding and every 48 h thereafter. All reagents were proven functionally effective in paralleled human umbilical vein EC studies (Fig. S1 and data not shown).
Files in this Data Supplement:
- Table S1. Primers used for Q-RT-PCR as described in materials and methods (PDF, 44.2 KB)
- Figure S1. S1P-induced SK activity in HUVEC inhibited by blocking receptors S1P1–3 (JPG, 44.9 KB) -
Receptor inhibitors pertussis toxin (PTX), JTE-013 (JTE) and VPC23019 (VPC) were administered to HUVEC 10 min prior to 30 min S1P exposure and cell lysis. An enzymatic assay identified SK activity between sample groups with experiments showing the mean ± sem from 3 separate HUVEC lines and *, p
- Figure S2. Increased adhesion of SK-1-KO ECs to fibronectin (JPG, 23 KB) -
WT and SK-1-KO mBM cells were cultured in CS-FCS containing media for 6 days prior to seeding onto Fn for 60 min. Adherent cells were counted and results represent total number of cells per well with the mean ± sem of 3 mice. *, p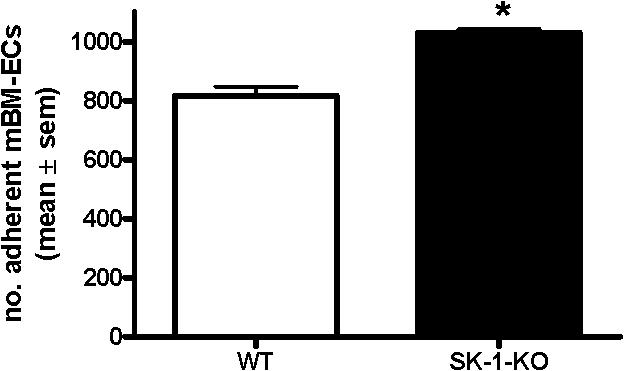
- Figure S3. SK activity decreases with time in culture (JPG, 24.3 KB) -
WT and SK-1-KO BM were cells cultured on Fn-coated plates for 3 and 11 days in CS-FCS containing culture media prior to SK activity being determined. Results of enzymatic activity normalized to 1 for WT day 3 and shown as the mean ± sem from 5 experiments with *, p
- Figure S4. Manipulation of SK-1 levels alters CD34 and VEGFR2 expression by HUVEC (JPG, 26.2 KB) -
HUVEC (2 × 106) were infected with adenovirus containing either control EV (HUVECEV) or SK-1 (HUVECSK) cDNA and cultured for 4 days. Flow cytometry and RT-PCR were used to determine CD34 surface expression and VEGFR2 mRNA levels, respectively. Experiments show the mean ± sem from 3 separate HUVEC lines and *, p