Blood, Vol. 113, Issue 10, 2256-2264, March 5, 2009
A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection
Blood Quigley et al. 113: 2256
Supplemental materials for: Quigley et al
Files in this Data Supplement:
- Figure S1. WT, MyD88−∕−, and TLR2−∕− CD8 T cells behave similarly in RAG-2−∕− mice (JPG, 122 KB) -
(A) Splenocytes from naïve WT, MyD88−∕−, and TLR2−∕− mice were stained with anti-CD8, anti-CD44, and anti-CD62L (top panels), or anti-CD8, anti-CD25, and anti-CD69 (bottom panels) antibodies. FACS plots are gated on CD8+ T cells and percentages shown are of those cells possessing an activated phenotype of CD44highCD62Llow or CD25highCD69high. (B–D) 1 × 106 purified WT, MyD88−∕− or TLR2−∕− CD8 T cells were transferred along with 2 × 106 WT polyclonal CD4 T cells into RAG-2−∕− hosts. 7 days after transfer, splenocytes were stained with anti-CD8, anti-CD44, and anti-CD62L (top panels), or anti-CD8, anti-CD25, and anti-CD69 (bottom panels) antibodies. Events are gated on CD8+ T cells, and percentages shown are of those cells possessing an activated phenotype of CD44highCD62Llow or CD25highCD69high (B). Splenocytes were also stimulated with 100 ng/ml PMA and 250 ng/ml ionomycin, and stained for IFN-γ intracellularly. Events are gated on CD8+ T cells, and percentages of IFN-γ producing CD8 T cells are shown (C). The absolute CD8 T-cell numbers per spleen are shown with standard deviations indicated (n=4 per group). MyD88−∕− or TLR2−∕− vs WT, p > 0.05 (D). Data shown are representative of two independent experiments.
- Figure S2. MyD88−∕−and TLR2−∕−Clone 4 T cells traffic similarly to their wild-type counterparts (JPG, 116 KB) -
1 × 104 naïve WT, MyD88−∕− or TLR2−∕− Clone 4 CD8 T cells (Thy1.1+) were transferred into B10.D2 mice that were subsequently infected with 5 × 105 pfurVV-HA intraperitoneally. 7 days after infection single cell suspensions were made from mesenteric lymph nodes (Mesenteric LN), lung (Lung), and liver (Liver), and stained with anti-CD8 and anti-Thy1.1 antibodies. The percentage of clonotypic T cells among total lymphocytes is shown. Data shown are representative of three independent experiments.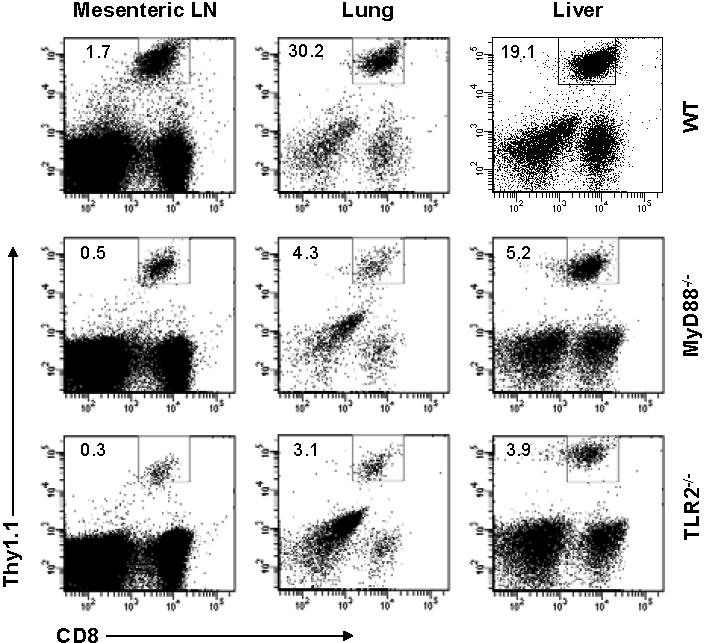
- Figure S3. Initial T-cell frequency does not effect defective clonalexpansion of CD8 T cells in the absence of MyD88 signaling (JPG, 61.7 KB) -
500 naïve WT or MyD88−∕− Clone 4 CD8 T cells (Thy1.1+) were transferred into B10.D2 mice that were subsequently infected with rVV-HA intraperitoneally. Recipient mice receiving no transgenic T cells (No transfer) infected with rVV-HA were used as a control. (A) Splenocytes were harvested at day 7 post-infection and stained with anti-CD8 and anti-Thy1.1 antibodies. The percentage of clonotypic T cells among total lymphocytes (top row) is indicated. Cells were also subjected to intracellular staining to determine the percent of IFN-γ–producing clonotypic T cells among total CD8 T cells (bottom row), with the percent of IFN-γ+Thy1.+CD8+ (Transgenic) T cells indicated in the top right quadrant, and the percent of IFN-γ+Thy1.−CD8+ (endogenous) T cells indicated in the bottom right quadrant. (B) The absolute cell numbers of Thy1.+CD8+T cells (left), IFN-γ+Thy1.+CD8+ T cells (middle), and IFN-γ+Thy1.−CD8+ T cells (right) per spleen for each respective group are shown with standard deviations indicated (n=4 per group). For transgenic T cells: MyD88−∕− vsWT, p−∕− vs No transfer, p> 0.05. Data shown are representative of two independent experiments.
- Figure S4. TLR expression in naïve and activated CD8 T cells (JPG, 69.3 KB) -
(A) Naïve CD8 T cells were purified from B10.D2 mice by two rounds of positive selection using anti-CD8 microbeads. The percentage of CD3+CD8+ T cells before (Pre-selection) and after (Post-selection) selection is indicated. (B) Total RNA was isolated from purified naïve (Naïve) or in vitroanti-CD3 and anti-CD28–stimulated (Activated) CD8 T cells. One-step semi-quantitative RT-PCR was performed using 5-fold serial dilution of template RNA and primers specific for murineTLR2, 3, 4, 7, 8, and 9 as well as β-actin as a loading control.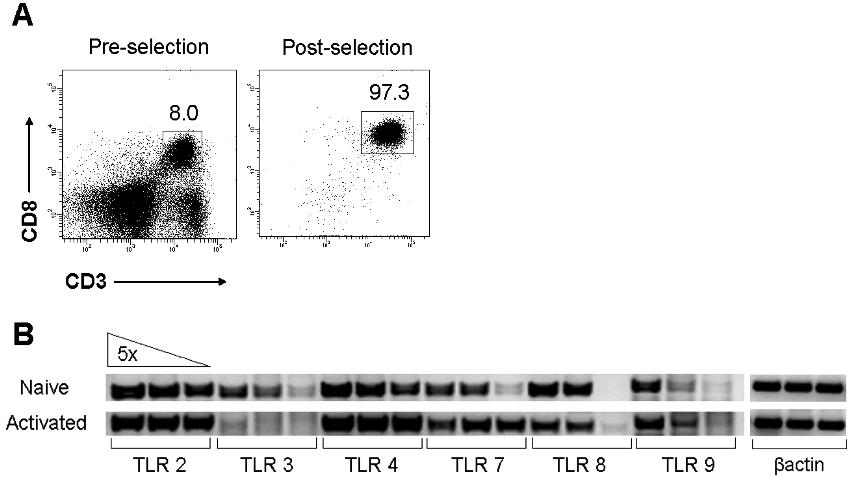
- Figure S5. CD28-mediated enhancement of CD8 T-cell survival is independent of the PI3K pathway (JPG, 132 KB) -
Purified CD8 T cells were stimulated in vitro with plate-bound anti-CD3 antibody alone (αCD3), anti-CD3 antibody coupled with plate-bound anti-CD28 (αCD3 αCD28), anti-CD3 with Pam3 Cys (αCD3 Pam3 Cys), anti-CD3, anti-CD28, and Pam3 Cys (αCD3 αCD28 Pam3 Cys), anti-CD3, Pam3 Cys, and the PI3K inhibitor LY294002 (αCD3 Pam3 Cys LY294002), anti-CD3, anti-CD28, and LY294002 (αCD3 αCD28 LY294002) or left unstimulated (Naive) as a control. After 18 hr of stimulation, T cells were removed from stimulation and placed back into culture in the absence of further stimulation for a total of 4 days. The survival of CD8 T cells was determined by Annexin V staining. Percentage of Annexin V− cells among total CD8 T cells is indicated. Representative data from two independent experiments is shown.
- Figure S6. Addition of multiple TLR ligands increases cellular proliferation and survival following in vitro stimulation (JPG, 46.3 KB) -
Polyclonal CFSE-labeled WT CD8 T cells were stimulated in vitro with plate-bound anti-CD3 antibody alone (αCD3), or anti-CD3 and Pam3 Cys (αCD3 Pam3 Cys), LPS (αCD3 LPS), or CpG (αCD3 CpG), or left unstimulated (Naive) as a control. After 18 hr of stimulation, T cells were removed from stimulation and put back into culture in the absence of further stimulation for a total of 4 days. At this time both the CFSE profile (upper panels) as well as the survival of CD8+ T cells by Annexin V staining (lower panels) was determined by flow cytometry. Percentage of Annexin V− cells amongst total CD8+ T cells is indicated.