Blood, Vol. 113, Issue 13, 2924-2933, March 26, 2009
SHIP is required for a functional hematopoietic stem cell niche
Blood Hazen et al. 113: 2924
Supplemental materials for: Hazen et al
Files in this Data Supplement:
- Figure S1. Schematic illustration of transplantation models (JPG, 81.1 KB) -
(A) In situ SHIP deletion BM transplants: MxCreSHIPflox∕flox(CD45.2+) 5 × 105 WBM cells were co-transplanted (i.v.) with 5 × 105 WBM competitor cells from WT-Ly5.1(CD45.1+) mice into lethally irradiated CD45.1+45.2+ recipients. Prior to transplant the recipients received a split dose of 11-Gy. After 60 days PB was used to monitor donor engraftment. Subsequently recipient mice were treated with three poly I:C injections to delete SHIP in all cells derived from the MxCreSHIPflox∕flox graft. (B) Systemic SHIP deletion BM transplants: MxCreSHIPflox∕flox(CD45.2+) mice were pre-treated with three poly I:C injections to systemically delete SHIP. After 21 days SHIP deletion was confirmed by Western blot analysis of PBMC. Once deletion was confirmed, 5 × 105 WBM cells from MxCreSHIPflox∕flox(CD45.2+) mice were co-transplanted (i.v.) with 5 × 105 WBM competitor cells from WT-Ly5.1(CD45.1+) mice into lethally irradiated CD45.1+45.2+ recipients. All recipients received a split dose of 11-Gy at least 2-hours before transplantation.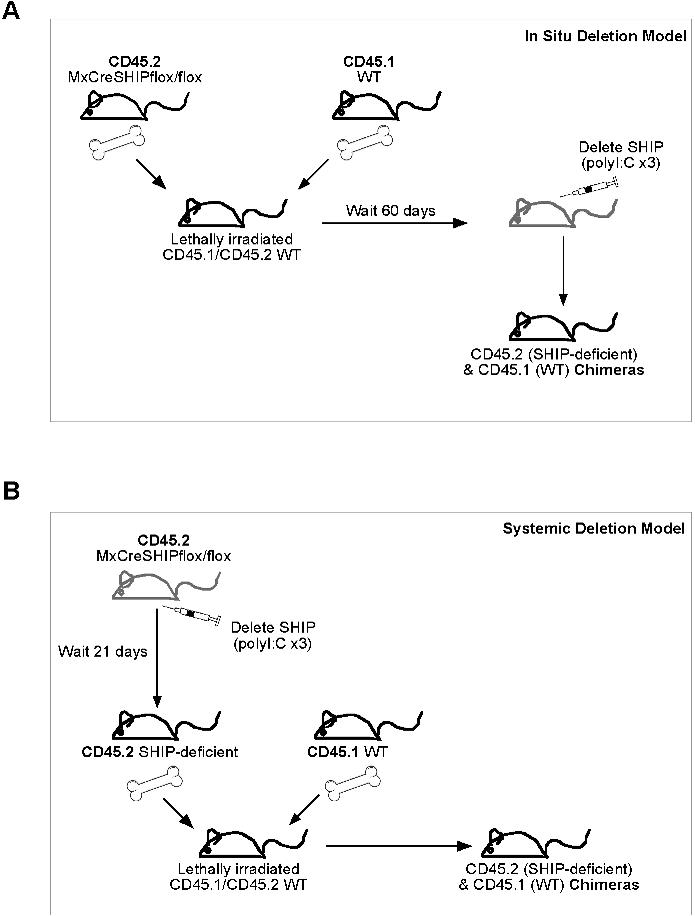
- Figure S2. SHIP−∕− OB cultures exhibit elongated and non-randomly oriented protrusions and organized growth along axes (JPG, 77.3 KB) -
Representative OB cells propagated from SHIP−∕− and WT femurs after 11, 13, and 23 days in culture. All images were generated using a Nikon TE2000 inverted microscope with a 10 × 0.25NA PlanFluor objective lens and a Retiga 1300 12bit CCD QImaging camera. The images were acquired using IP Lab v3.6 software (Scanalytics of BD Biosciences).
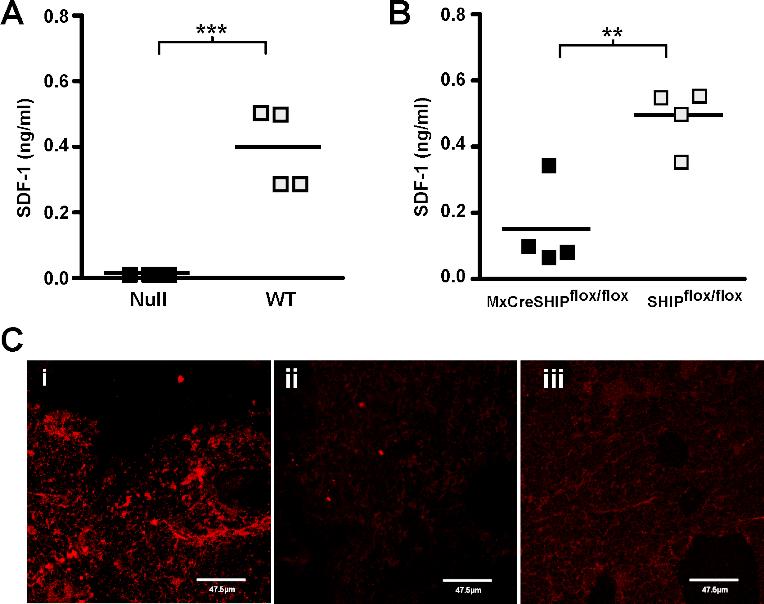
- Figure S3. SHIP-deficiency impairs SDF-1 production in the BM niche (JPG, 77.5 KB) -
(A) Scatter plots indicating the mean and range for the concentration of SDF-1/CXCL12 in the cell culture supernatant of SHIP−∕− and WT BM stromal cells. Results are representative of independent experiments on stromal cultures prepared from three independent pairs of SHIP−∕− and WT mice. (B) Scatter plots indicating the mean and range for the concentration of SDF-1/CXCL12 in the cell culture supernatant of MxCreSHIPflox∕flox and SHIPflox∕flox BM stromal cells from polyI:C treated mice. Results are representative of independent stromal cultures derived from independent mice. (C) Representative photomicrographs (63×) of frozen sections prepared from adult femurs of WT (i) and SHIP−∕− mice (ii) that were stained with biotinylated mouse anti–SDF-1 Ab (MAB350, R&D Systems) (i, ii) or a biotinylated mouse IgG1k control antibody (iii) (MOPC-31C, Becton Dickinson). Staining by the anti–SDF-1 or the IgG1k control was revealed by a secondary stain of SA-AlexaFluor 546 (Molecular Probes). The background SDF-1 staining observed with SHIP−∕− BM sections was consistently comparable to staining observed in isotype control stains performed on both SHIP−∕− and WT femurs (iii and data not shown). Significance was established using the unpaired Student’s T test (**p−∕− samples(A); Grey, WT samples(A); Black, MxCreSHIPflox∕flox samples(B); Grey, SHIPflox∕flox samples(B).