© RSNA, 2009
PROSE
The point-resolved spectroscopy (PRESS) sequence (PROSE) is a double-spin-echo sequence that uses a 900 excitation pulse followed by two section-selective spectral spatial refocusing pulses combined with three-dimensional phase-encoding gradients to generate chemical shift images of multiple voxels within a prescribed volume (13,14,17,18). The PRESS parameters were as follows: repetition time = 1 second, echo time = 130 msec, number of excitations = 1, phase-encoding steps = 16 × 8 × 8, field of view = 110 × 55 × 55 mm, acquisition time = 17 minutes.
Several aspects of the PROSE package merit specific description. The high-bandwidth spectral spatial refocusing pulses in the PRESS sequence were designed to provide both accurate volume localization and frequency selection (19). Specifically, the high spectral bandwidth minimized chemical shift artifacts at the edges of the selected volume, and the frequency characteristics of the pulses provided full excitation of the prostate metabolites (choline, creatine, and citrate) while fully suppressing the lipid region of the spectrum and partially (≈10%) exciting water. Selection of the spectroscopic imaging volume was done by drawing a rectangle at the prostatic apex and base on the axial T2-weighted images, maximizing coverage of the prostate while minimizing inclusion of periprostatic fat. Seminal vesicles were excluded from the spectroscopic volume when possible. The PROSE package then automatically determined optimal position and dimensions for the volume and was aligned to the oblique-axial plane of the T2-weighted imaging from which the PROSE box was prescribed. Magnetic field homogeneity was optimized for the selected volume by using an automated shimming algorithm within the package. The prescribed volume was automatically overexpanded (by 10% in the anteroposterior direction and 30% in the transverse and craniocaudad directions), and very selective outer-voxel suppression pulses were automatically placed at the original boundaries of the selected volume to further improve spectral localization and reduce contamination from surrounding tissues (20). Additionally, the supervising technologist had the ability to graphically place up to six more (four on an axial image and two on a sagittal image) very selective outer-voxel suppression pulses to better conform the rectangular selected volume to the globular shape of the prostate.
Data and Image Handling
Data were collected and reviewed by using Web-based electronic forms stamped with the date and time of receipt by the ACRIN server, with the exception of the reader and pathology forms, which were manually entered by Data Management Center personnel. Validation programs were used to perform data checks for accuracy and completeness as data were collected. The Data Management Center provided routine reports to each participating institution, identifying patients with missing information and clarifying inconsistencies in information. Further data inspection commenced as data were transferred to the Biostatistics Center. Errors and missing responses were queried according to the standard ACRIN data collection process. The study was monitored by the ACRIN Data Safety Monitoring Board. All images were provided in digital format. ACRIN software allowed electronic transmission to the image archive, with all patient identifiers removed. Patient identifiers were also removed from the DICOM format spectral arrays output by the PROSE package. Individual computers with this software were supplied to each participating site. Images stored in the ACRIN archive were digitally routed to other sites for secondary interpretation.
Rationale for Sextant-By-Sextant Analysis
Our sextant-by-sextant approach is substantially different from a design that simply examines the accuracy with which readers can identify a positive node or focus. A per-nodule methodology would have allowed use of alternative free-response receiver operating curve (AFROC) analysis (47). In a per-node analysis, only the true-positive and false-positive rates are considered and readers are not required to evaluate the same nodes. Because readers select the nodes to bring up, they can inflate their accuracy and often reduce their generalizability because of selection bias. In addition, AFROC analysis cannot be used to inform the user about the diagnostic accuracy with which MR spectroscopy could help detect cancer occult at MR imaging alone. That is, the sensitivity-specificity trade-off, and hence overall accuracy, is not assessed with an AFROC analysis because negatives are essentially ignored. Moreover, it stands to reason that if readers cannot localize or detect cancer in a predefined area of the prostate, they will have a difficult time accurately idenifying individual nodes in that predefined area.
Another analytic option would have been to select voxels that consist of benign or malignant tissue on the basis of correlation with histopathology and then have readers evaluate only those voxels. Such an approach may help tease out the scientific benefit of MR spectroscopic imaging for tissue characterization (23,32) but is probably flawed with respect to evaluating the real-life clinical benefit. On balance, the receiver operating characteristic analysis is representative of the accuracy with which readers can localize and detect cancer in the prostate.
Reference
47. Chakraborty DP, Winter LH. Free-response methodology: alternate analysis and a new observer-performance experiment. Radiology 1990;174:873-881.
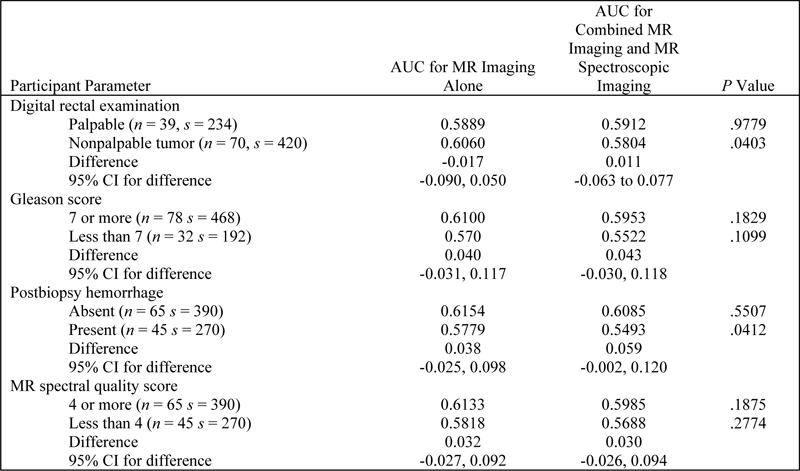
Table E1. AUC for Subgroups based on Participant Characteristics

Note.—CI = confidence interval, n = number of participants, s = number of sextants.
Table E2. Sensitivity for Subgroups based on Participant Characteristics
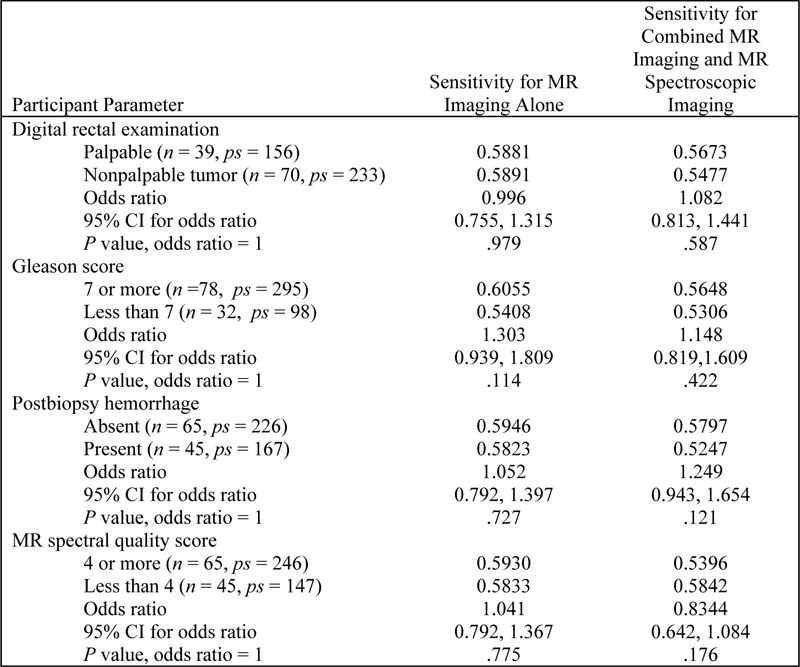
Note.— CI = confidence interval, n = number of participants, ps = number of positive sextants.
Table E3: Sensitivity for Subgroups based on Sextant Characteristics
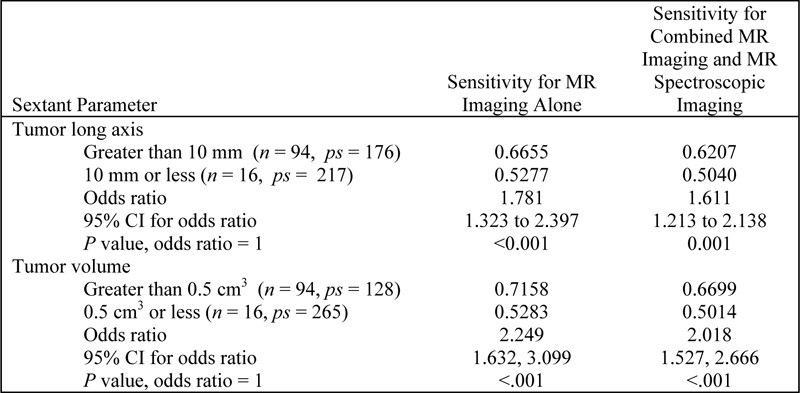
Note.— CI = confidence interval, n = number of participants, ps = number of positive sextants.