Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults
Sjoerd Zwart, Alfred P E Sachs, Gijs J H M Ruijs, Jan W Gubbels, Arno W Hoes, Ruut A de Melker
Julius Center for General Practice and Patient-oriented Research, University Medical Center Utrecht, 3584 CG Utrecht, NetherlandsSjoerd Zwart
senior researcher
Alfred P E Sachs
senior researcher
Arno W Hoes
professor of clinical epidemiology
Ruut A de Melker
formerly professor of general practice
Gijs J H M Ruijs
clinical microbiologist
Jan W Gubbels
statistician
Correspondence to: S Zwart s.zwart@med.uu.nl
Abstract
Objective To assess whether treatment with penicillin for three days and the traditional treatment for seven days were equally as effective at accelerating resolution of symptoms in patients with sore throat compared with placebo.
Design Randomised double blind placebo controlled trial.
Setting 43 family practices in the Netherlands.
Participants 561 patients, aged 15-60 years, with sore throat for less than seven days and at least three of the four Centor criteriaC that is, history of fever, absence of cough, swollen tender anterior cervical lymph nodes, and tonsillar exudate. 142 patients were excluded for medical reasons and 73 needed penicillin.
Interventions Patients were randomly assigned to penicillin V for seven days, penicillin V for three days followed by placebo for four days, or placebo for seven days.
Main outcome measures Resolution of symptoms in the first week, eradication of bacteria after two weeks, and recurrences of sore throat after two, four, and six months.
Results Symptoms resolved 1.9 and 1.7 days earlier in patients taking penicillin for seven days than in those taking penicillin for three days or placebo respectively. Symptoms resolved 2.5 days earlier in patients with group A streptococci and 1.3 days earlier in patients with high colony counts of non-group A streptococci. 23 (13%) of the placebo group had to be given antibiotics later in the week because of clinical deterioration; three developed a peritonsillar abscess. The eradication rate for group A streptococci was 72% in the seven day penicillin group, 41% in the three day penicillin group, and 7% in the placebo group. Sore throat recurred more often in the three day penicillin group than in the seven day penicillin or placebo groups.
Conclusion Penicillin treatment for seven days was superior to treatment for three days or placebo in resolving symptoms of sore throat in patients with group A streptococcal pharyngitis and, possibly, in those with non-group A streptococcal pharyngitis.
Introduction
At least once a week a general practitioner is confronted with a patient with an acute sore throat. Antimicrobial treatment is usually unnecessary because most of the infections are of viral origin.
Penicillin has been the drug of choice for the treatment of group A b haemolytic streptococci pharyngitis for more than four decades because it accelerates the resolution of symptoms and reduces the number of suppurative complications. (1) Penicillin is also considered superior to its alternatives because of lack of resistance, fewer adverse effects, and lower costs. (2) Prevention of acute rheumatic fever is no longer the main reason to treat patients with penicillin in western Europe, because of the low incidence of this complication. (3)(4) (5)
Traditionally a regimen of penicillin for 10 days has been advocated to maximise eradication of bacteria. (2)(6) Three studies have also shown that shortening the duration of penicillin treatment increases the incidence of bacteriological, but not clinical, recurrences. (6) (7)(8) As a compromise between the established regimen of 10 days treatment with penicillin and the newer five days treatment,which is already common practice in the Netherlands, (9) national guidelines in the Netherlands now advocate the treatment of group A streptococcal pharyngitis for seven days. (10) Empirical evidence for this recommendation is, however, lacking. In most trials symptoms of sore throat disappeared within three or four days of antibiotic treatment. Moreover, treatment of other upper respiratory tract infections for three and five days have proved effective, (11) (12) whereas treatments exceeding five days have shown Streptococcus pneumoniae to develop resistance to b lactam antibiotics. (13)
We hypothesised that the resolution of symptoms of sore throat would not be influenced by shortening the traditional duration of penicillin treatment. We included a placebo group for comparison with the clinical course of the disease. We compared the clinical and bacteriological effects of penicillin treatment for seven days with treatment for three days and placebo in adults with acute sore throat selected by the clinical criteria according to the Dutch guidelines.
Participants and methods
Participating practices
Our study was conducted from 1994 to 1996. We invited 90 general practitioners from 71 practices to participate, 55 general practitioners from 43 practices in the towns of Zwolle and Kampen and the surrounding predominantly rural area of the Netherlands agreed to participate and 35 refused for various reasons.
Patients
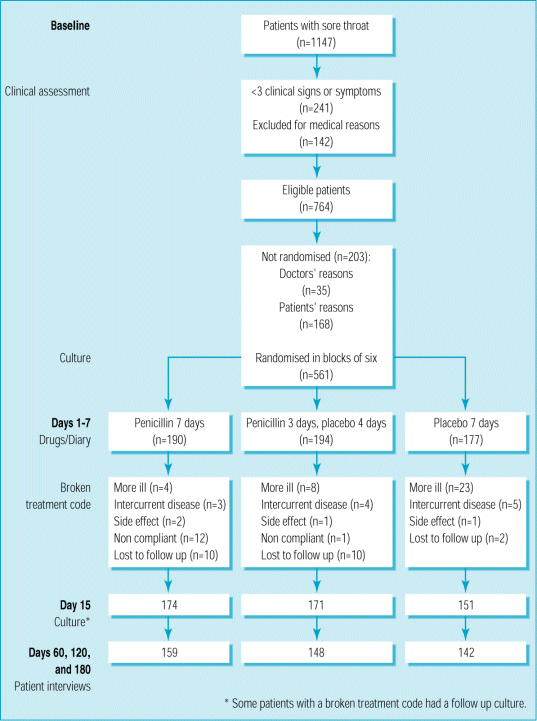
Overall, 1147 patients aged 15-60 years contacted their general practitioner because of an acute (seven days or less) sore throat (fig 1). We excluded 241 (21.0%) of these because they presented with fewer than three of the four Centor criteriaC that is, history of fever, absence of cough, swollen tender anterior cervical lymph nodes, and tonsillar exudate. (14) These criteria were validated against the results of throat cultures from an emergency room setting in North America (adults only) (14) and from primary care settings (children and adults) in both Holland (15) and Scandinavia. (16) The prevalence of group A streptococci in adult patients was around 45% in the first two studies but could not be extrapolated from the third study.
Figure 1

Of the 906 patients who fulfilled the Centor criteria, 142 (15.7%) were excluded: 9 (6%) had had antibiotics in the previous two weeks, 13 (9%) had suspected intolerance to penicillin, 21 (15%) had suspected infectious mononucleosis, 26 (18%) were unable to comprehend the protocol or diary, and 73 (51%) needed penicillin urgentlyC imminent quinsy (68 patients), history of glomerulonephritis (1), immunocompromised (4). Of the 764 eligible patients, 203 (26.6%) were not randomised for various reasons (fig 1). The non-randomised patients presented with the four Centor criteria more often than the 561 included patients. Neither the non-randomised patients nor the 241 patients who met only one or two Centor criteria differed from the 561 included patients for age, sex, and type of healthcare insurance. Informed consent was obtained from each patient. The study protocol was approved by the medical ethics committee of the Isala Clinics, Zwolle.
Clinical evaluation at baseline
Along with the Centor criteria, information was obtained on duration of sore throat, absence from school or work, history of tonsillectomy, smoking habits, number of household members, type of healthcare insurance (private or collective, as an indicator of socioeconomic status), and urbanisation (30 000 or less inhabitants or more than 30 000 inhabitants; table 1). The number of episodes of pharyngitis and other upper respiratory tract infections as diagnosed by the general practitioner in the previous six months was also recorded. Upper respiratory tract infections were categorised as pharyngitis, acute otitis media, sinusitis, or "unspecified."
Table 1 Reasons to exclude patients. Number and percentage of all patients excluded (N=142)
| Antibiotic use in the previous 2 weeks | |||
| (Suspected) intolerance to penicillin | |||
| (Suspected) infectious mononucleosis | |||
| Unable to comprehend protocol/diary | |||
| Urgent need of penicillin | |||
| (imminent) quinsy* | |||
| history of glomerulonephritis | |||
| immunocompromized | |||
| Total |
*(Imminent) quinsy: Unilateral (peri-) tonsillar swelling with edema and exudate, pushing aside the uvula.
Treatment groups
Patients were randomly assigned (according to a computer generated list of six numbers per block, blinded to doctors and patients) to one of three treatment groups: penicillin V for seven days, penicillin V for three days followed by placebo for four days, or placebo for seven days. The dosage was two 250 mg capsules three times daily. The placebo capsules (1 mg quinine added to lactose powder) had the same appearance and taste as the penicillin capsules. Paracetamol tablets were supplied to all patients to be used on demand. When requested by the patient or doctor the study coordinator (SZ) broke the code of treatment.
Clinical follow up
The patients kept a diary during the treatment period. Nightly they recorded the extent of throat complaints, the degree of impairment of daily activities (both on a five point categorical scale), and their oral temperature. They also recorded the number of analgesics used daily (as an additional indicator of clinical response) and whether nausea, diarrhoea, abdominal pain, and skin rash were present (possible adverse effects of the drug).
Fourteen days after inclusion the patients were re-examined by their general practitioner and were asked about throat complaints in the second week when no diary was kept. After two, four, and six months the patients were interviewed by telephone. Questions were asked regarding sore throat and other complaints possibly related to an upper respiratory tract infection: cough, runny nose, and earache. If a patient returned during the six month follow up period two throat samples were taken for culture. After six months the general practitioner recorded the total number of encounters with the patient and any upper respiratory tract infection or complication diagnosed in the preceding six months.
Bacteriological measurements
The general practitioners were trained to take throat samples in an identical manner, thoroughly swabbing both the tonsils or tonsillar fossae and the posterior pharyngeal wall. Two subsequent samples were taken from each patient at the initial visit and again at the follow up visit after 14 days, because previous research has shown that the bacteriological yield from two samples is higher than the yield from one sample. (17) The samples were then transported in a modified Stuart medium and treated in the laboratory within 48 hours of sampling.
At the laboratory each throat swab was rolled semiquantitatively onto a 5% sheep blood and an ssA agar plate. The plates were incubated anaerobically at 35°C for up to 48 hours. b Haemolytic colonies that were catalase negative and showed Gram positive cocci in chains were serogrouped using Streptex (Murex Diagnostics, Utrecht, the Netherlands). Colony counts of b haemolytic streptococci were reported in a semiquantitative manner: no growth, sporadic colony counts (1-10 colonies), 1+ or low colony counts (more than 10 colonies but limited to initial inoculation area), 2+ or medium colony counts (growth into second inoculation area), and 3+ or high colony counts (growth into third inoculation area). Susceptibility to penicillin was tested by disk diffusion (Neosensitabs, Zutphen, the Netherlands). When two different serotypes were found in one culture only the serotype with the highest number of colony counts was included in the analysis. The serogroup A isolates were stored at - 85°C for T and M typing at the National Institute of Public Health and the Environment in Bilthoven.
Outcome measures
The primary outcome variable was the duration of symptoms, defined as the number of days until permanent resolution of either pain or impaired daily activities took place (both "absent" on the 5 point scale in the diary). Thus, in case of reappearance in the first week the duration was calculated at the day permanent resolution was recorded in the diary. For example, if a patient was symptom free at day 3 but developed symptoms again at day 4 or 5, which resolved at day 6 or 7, the duration was calculated as five days. Secondary outcome variables included the occurrence of post streptococcal complications and recurrent episodes of sore throat or any other upper respiratory tract infection in the following six months. The bacteriological outcome variable was the eradication of the initial pathogen.
Compliance
Drug compliance was measured by checking the number of swallowed capsules recorded in the diary and the number of capsules left in the tray, which was returned to the practice after 14 days. The patient was defined as non-compliant if more than one penicillin dosage was left in the tray for the three day regimen or more than two dosages were left in the tray for the seven day regimen, as indicated by the patient or practice assistant.
Sample size estimation
Assuming a minimum absolute difference of 20% with regard to the proportion of patients with resolution of symptoms at days 3-7 between each pair of the three treatment groups to be relevant, the required number of patients (a =5%, b =10%) was 122 in each group. To allow for a separate analysis of patients positive for b haemolytic streptococci (estimated to be 66% of all included patients) (15) we aimed at including 185 patients in each of the three treatment groups.
Data analysis
All analyses were carried out with spss version 7.0, using an intention to treat approach. Categorical differences between the three treatment groups were tested using the c2 test (with continuity correction) and Fishers’ exact test (for small numbers). Kaplan-Meier curves depicting duration of sore throat and impaired daily activities were plotted and the differences were tested with the Wilcoxon (Gehan) statistic. Multiple Cox regression analyses were performed to adjust for confounding factors. The Wald statistic in Cox regression was used to study modification of the treatment effect by patient characteristics at baseline, such as age, season, sex, number of household members, and duration of complaints. The survival analyses were repeated for those 475 patients in whom the treatment code was not broken to compare the results of the intention to treat analysis with an on treatment analysis.
Results
A comparison of patients from the 19 general practitioners who recruited more than 10 patients each with those from lower recruiters showed similar proportions of males, early attenders, patients with four clinical signs and symptoms, and patients with positive culture results for group A streptococci.
Resolution of symptoms
Patients who took penicillin for seven days showed a permanent resolution of sore throat 1.9 and 1.7 days sooner than those who took penicillin for three days or placebo respectively (fig 2 and table 2). During the first three days of treatment, patients in the three day penicillin group showed a similar resolution of symptoms to those in the seven day penicillin group. However, 40% (77 of 194) of the three day penicillin group had a temporary resolution of symptoms, which recurred later that week, against 5% (10 of 190) of the seven day penicillin group. This finding accounts for the difference between the two penicillin groups in the Kaplan-Meier curves during the first three days (fig 2). Using the definition of permanent resolution of symptoms, patients in the three day group did not recover more rapidly than those in the placebo group. Analgesic use until day 4 was similar in all three groups. From day 1 until day 7, however, the proportion of patients taking analgesics declined from 61% to 5% in the seven day penicillin group, whereas in the two other treatment groups the reduction from day 4 until day 7 was considerably smaller.
Table 2 Baseline characteristics of sore throat patients per treatment group*
(n=190) | (n=194) | (n=177) | |
| Mean age (SD), y | |||
| Sex (% male) | |||
| Health Care Insurance (% Public Health Plan) | |||
| >3 household members | |||
| Urbanization (>30 000 inhabitants) | |||
| Season (October-March) | |||
| Medical history | |||
| History of tonsillectomy | |||
| Smoker (>4 cigarettes/d) | |||
| Sore throat previous 6 m† | |||
| URI previous 6 m† | |||
| Clinical presentation | |||
| Sore throat >3 days | |||
| Absence from school/work‡ | |||
| Fever (reported) | |||
| Absence of cough | |||
| (Tonsillar) exsudate | |||
| Anterior cervical lymph nodes | |||
| All 4 Centor criteria present | |||
| Streptococci positive culture | |||
| Group A | |||
| Group C | |||
| Group G | |||
| Other serogroups | |||
| Streptococci negative culture | |||
*All values are percentages except age.
†At least one patient-doctor encounter for this reason in the previous 6 months.
URI = upper respiratory tract infection.
‡Calculated for those who should have attended school or work that day.
Figure 2
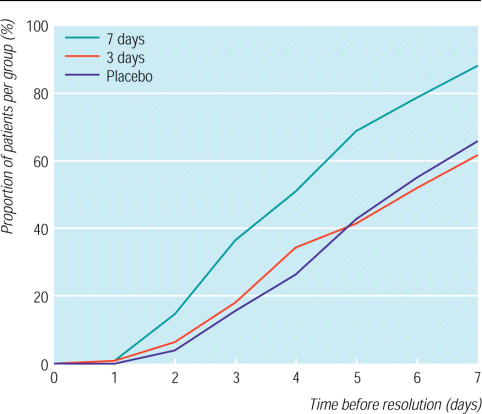
Kaplan-Meier plot for resolution of symptoms of sore throat in patients treated with penicillin for seven or three days or placebo
Penicillin treatment for seven days was most effective in relieving symptoms in patients with group A streptococci and also effective in those with 3+ non-group A streptococci (table 2). Patients with negative culture results for streptococci also showed a clinical response to penicillin although the difference was not statistically significant (table 2). Older age (over 27 years) increased the effect of penicillin on the resolution of sore throat, whereas the presence of four Centor criteria, a history of tonsillectomy, and a short duration of sore thoat (three or less days) at baseline did not have any influence.
Patients who took penicillin for seven days resumed their daily activities 2.2 and 2.0 days earlier than those in the three day penicillin group or placebo group respectively (fig 3). The reattendance rate of the 267 patients who were initially unable to attend school or work was, however, similar in the three treatment groups.
Figure 3
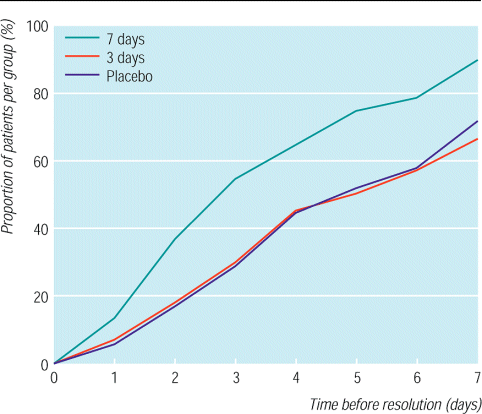
Kaplan-Meier plot for resolution of impaired daily activities in patients treated with penicillin for sore throat for seven or three days or placebo
Six patients treated with placebo had a streptococcal complication: three had peritonsillar abscesses (streptococci groups A 3+, B 3+, and G3+), one had erysipelas of the hand, one had impetigo, and one had transient polyarthritis (these last three patients were positive for group A streptococci 3+; no serology was carried out). Acute rheumatic fever and post streptococcal glomerulonephritis did not occur. The treatment code had to be broken because of persisting pain, imminent abscess, or a complication in four (2%) patients treated for seven days (two had group A streptococci), eight (4%) patients treated for three days (four had group A streptococci), and 23 (13%) patients treated with placebo (16 had group A streptococci) (fig 1).
Bacteriological response
At baseline 442 (78.8%) of the 561 patients had a positive culture result for b haemolytic streptococci. Group A streptococci were cultured in 280 (49.9%) patients, group C in 67 (11.9%), group G in 44 (7.8%), group B in 33 (5.9%), group F in 12 (2.1%), and non-typeable streptococci in 6 (1.1%); all strains were susceptible to penicillin. Two different serotypes were found in 8 (1.4%) patients. Patients who had had tonsillectomies had a lower prevalence of b haemolytic streptococci (70%, 74 of 105) than those with tonsils (81%, 368 of 456). The prevalence of b haemolytic streptococci was similar in patients with three and four Centor criteria. Overall, 474 (84.5%) patients returned for the follow up culture after two weeks (range 12-20 days, mean 15 days). Treatment with penicillin for seven days was more effective than treatment for three days in eradicating group A and group G streptococci but not group C streptococci from the throat (table 3).
Table 3 Shortening of the median duration of sore throat (no. days). Pairwise comparison* of 7-day penicillin treatment with 3-day and 0-day penicillin treatment per throat culture result (beta-hemolytic streptococci)
| Throat culture | ||||||||
| All | ||||||||
| Group A | colony counts | |||||||
| Non-Group A | colony counts | |||||||
| Negative | ||||||||
*Wilcoxon (Gehan) analysis.
Recurrences
From the second week until six months after inclusion in the study the incidence of recurrent episodes of sore throat was similar in the seven day penicillin group and placebo group (around 30%) and higher in the three day penicillin group (40%). This difference, however, had no impact on the reattendance rates (table 4). Six patients underwent tonsillectomy; four had a positive culture result for group A streptococci and two had a positive culture result for group C streptococci. Of the 44 patients who revisited their doctor because of a recurrent sore throat, 27 (61%) had a throat culture; they were more or less equally distributed among the treatment groups.
Adverse effects
Nausea occurred more often in the penicillin groups (40% (76 of 190) in the seven day group and 39% (76 of 194) in the three day group) than in the placebo group (16%, 28 of 177). Abdominal pain was also reported more frequently in the penicillin groups (25% (48) in the seven day group and 28% (54) in the three day group) than in the placebo group (15%). The presence of diarrhoea and skin rash was similar in all three treatment groups.
On treatment analysis
On treatment analysis showed a pattern similar to the intention to treat analysis shown in figure 2: the median duration of pain and of impaired daily activities was 1.5-1.9 days shorter in the seven day penicillin group than in the three day penicillin and placebo groups.
Discussion
In our study, penicillin treatment for seven days shortened the duration of both sore throat and impaired daily activities by about two days in adult patients with sore throat. This reduction is larger than that reported in earlier studies (1) and was seen not only in patients positive for group A streptococci but to a smaller extent in patients with a high colony count for non-group A streptococci.
Patient selection
The selection of our patients was based on clinical features and not the results of a rapid detection test for group A antigen because the management of sore throats in primary care in Holland is founded on clinical and not bacteriological grounds. (10) Furthermore, such a test would have excluded all patients with non-group A streptococcal pharyngitis.
The 106 patients who refused to participate because of their preference for penicillin treatment may have been more ill than those randomised because they met more Centor criteria. Since it seems reasonable to assume that the most seriously afflicted patients did not participate in the trial, inclusion of these patients would have contributed to an even more pronounced effect of penicillin.
Resolution of symptoms
The acceleration of resolution of symptoms by 2.5 days recorded in our patients positive for group A streptococci and treated with penicillin for seven days is unprecedented. We included a record number of adults, selected by a validated set of clinical criteria, and we used the endpoints of a complete and permanent resolution of sore throat and impaired daily activities. As far as we know only six placebo controlled trials in the past 50 years have assessed the effect of penicillin, given for either five (18) (19) (20) or 10 (3) (4) (5) days, on the resolution of sore throat in adults. In general, an acceleration of resolution of symptoms by 0.5-1.0 day was observed in the penicillin groups in these studies, notably in patients positive for group A streptococci. Unfortunately, we could not compare all of these trials with ours. For example, one study with an open randomised protocol did not include bacteriological assessments. (5) Another study included more than 200 adult patients, but only provided limited information on selection criteria and withdrawals (19); only 8% of their patients were positive for group A streptococci. A recent meta-analysis of 10 484 patients with sore throat reported that antibiotics shortened the duration of symptoms by about eight hours. (1) The wide variety of inclusion criteria notably age, bacteriological methods, and clinical endpoints, however, hampered comparison with our results.
Patients without group A streptococci
Our finding that treatment with penicillin for seven days was possibly effective in patients positive for non-group A streptococci supports suggestions in the literature about the causal role in non-group A streptococcal pharyngitis, (21) notably in patients with high colony counts. (22) (23) Our finding should, however, be interpreted with caution as the sample size calculation did not allow for subgroup analysis. To date only one other placebo controlled trial has studied patients positive for non-group A streptococci and found no effect from penicillin. (4) This may, however, be attributable to the small size of the subgroup.
We also observed a trend towards a clinical effect of penicillin in patients with a negative culture result for streptococci (table 2). Other researchers have found erythromycin (24)(25) and cefixime (20) effective in accelerating resolution of symptoms in non-streptococcal pharyngitis. Apparently, other microorganisms sensitive to penicillin, such as Staphylococcus aureus and Haemophilus influenzae, which we did not look for, may also play a causal role in acute sore throat.
Risks of penicillin for three days or placebo
Penicillin treatment for three days and placebo were not equally effective. The difference between the three day and seven day penicillin groups during the first three days of treatment (figs 2 and 3) is an artefact. Because of the definition "permanent resolution of symptoms" all patients showing a temporary resolution of symptoms during the first days of the week were considered to be treatment failures until the day that symptoms did not recur. Most treatment failures were in patients who took penicillin for three days. The three day penicillin group also tended to have an increased recurrence rate in the following six months (table 4). Although the reason for this trend is unknown it may well be that the short duration of penicillin treatment only reduced the natural immune response and suppressed the pathogenic streptococci without eradicating them, perhaps because they were sequestered in the epithelial cells of the respiratory tract. (26) This phenomenon may have been the reason for the unacceptable number of treatment failures in a recent Swedish trial of penicillin treatment for five days, which was terminated prematurely. (27)
Table 4 Eradication rate of the treatment groups, per homologous group A, group C and group G beta-hemolytic streptococci serogroup. Numbers (percentages)
| Group A | |||
| Group C | |||
| Group G | |||
*P<0.05 compared to placebo, and P<0.05 compared to 3 days penicillin (pairwise testing).
†P<0.05 compared to placebo.
The risk of recurrent sore throat in the seven day penicillin group was similar to that in the placebo group, a finding concordant with the other placebo controlled study that measured recurrences. (4) In another study, the medicalising effect of previous antimicrobial prescriptions was held responsible for the high reattendance rate in the penicillin group. (28)
We were confronted with six post streptococcal complications, including three abscesses, and a high percentage of patients in the placebo group who broke the treatment code owing to persisting or worsening complaints. The risk of an abscess was considerably reduced by antibiotics, a finding concordant with the relative risk of 0.19 calculated in a recent meta-analysis. (1) The reported adverse effects of penicillin were mild and the reason for only three patients (0.8%) dropping out.
Eradication of streptococci
The 72% eradication rate for group A streptococci in the seven day penicillin group (table 3) was lower than the 87%-91% eradication rate detected within two weeks after completion of a 10 day penicillin regimen in two studies of predominantly adult patients. (8)(29) Nevertheless, there is no evidence that a 10 day penicillin regimen reduces the risk of non-suppurative complications more effectively than a seven day or five day regimen. (1) We do not know why penicillin seemed to eradicate group G streptococci more effectively than group C streptococci (table 3). Group C and G streptococci tend to be more prone to penicillin tolerance than group A streptococci but not at a clinically relevant level. (30)
Conclusion
If the aim of penicillin treatment is the acceleration of resolution of symptoms and a reduced risk of suppurative complications, a seven day course of penicillin is the most effective treatment for adult patients with sore throat caused by group A streptococci and, possibly, non-group A streptococci. The surplus value of a 10 day course of penicillin was found in the literature only in terms of increased bacterial eradication. (6) Since the three day course of penicillin was not at all effective and a five day course probably insufficient the trend in western Europe to reduce the duration of treatment of streptococcal pharyngitis should be discouraged. Furthermore, we may have detected a new target group for penicillin treatmentC that is, patients with high colony counts for non-group A streptococcal pharyngitis. Further refinement of the current detection tests is needed to identify the infections caused by group A streptococci or non-group A streptococci within the wide spectrum of patients with sore throat consulting their doctor. (31) In view of the growing problem of bacterial resistance, prudent prescribing of penicillin is needed to minimise irrational expectations of patients and doctors towards antibiotics.(32)
We thank the participating patients, general practitioners and their assistants, Yvonne Mulder and Gerben Kajim for administrative assistance, the Associated Pharmacies Ysselmond in Kampen for preparing the study drugs, the Laboratory for Microbiology and Infectious Diseases in Zwolle and the National Institute for Public Health and the Environment (RIVM) in Bilthoven for technical assistance, Laura Cobb for correcting the English text, and Dr Carien Dagnelie, Professor Doeke Post, Dr Joop Schellekens, and the late Professor Fransje Touw for their contribution to the study design.
Contributors: SZ initiated the research, designed the protocol, collected and analysed the data, and wrote the paper. GJHM Ruijs, JWG and RAdeM were involved in the study design, funding, data collection, and analysis. APES and AWH were involved in the data analysis. All the authors participated in writing the paper. SZ will act as guarantor for the paper.
Funding: This study was funded by Groene Land Health Insurances (Achmea Group) and the Stichting Gezondheidszorgonderzoek Ysselmond in Zwolle. Boots Pharmaceuticals kindly supplied the paracetamol tablets.
Competing interests: None declared.
Read all Rapid Responses