Blood, Vol. 114, Issue 8, 1696-1706, August 20, 2009
Direct crosstalk between mast cell–TNF and TNFR1-expressing endothelia mediates local tissue inflammation
Blood Kneilling et al. 114: 1696
Supplemental materials for: Kneilling et al
Optical imaging
The presence of engrafted dermal Cy5-stained mast cells was investigated in vivo by optical imaging. First, mice were anaesthetized by inhalation of isoflurane-O2 (1.8%; Forene, Abbott GmbH, Wiesbaden, Germany) and placed on a heating pad to maintain body temperature between 36°C and 37°C. 0.5 × 106 (in 30 µl PBS) Cy5 labeled mast cells or 0.5 × 106 RFP labeled (control) mast cells were injected directly (intra-cutaneously) into the right ear of TNFR1−∕− mice 27 days prior optical imaging in vivo investigation. For in vivo investigations we used a Hamamatsu Aequoria Dark Box and a C4880 Hamamatsu Dual Mode cooled CCD camera (Hamamatsu Photonics Deutschland GmbH, Herrsching, Germany).
Red fluorescence protein (RFP) and Cyanine (Cy)5 staining of mast cells
RFP and Cy5 was performed according to the manufactures standard protocol. For Cy5 staining 2 × 106 mast cells/ml were incubated for 2 minutes (Vybrant™, Molecular Probes, Invitrogen™, Eugene, USA). For RFP-staining 7.5 × 106/ml mast cells were incubated for 5 minutes (PKH26 Fluorescent Linker Kit, Sigma Aldrich, Germany).
Western blot analysis
Ear tissue was harvested 2.5 h and 4.0 h after TNCB challenge and immediately homogenized in protein lysis buffer (2 mM EDTA, 0,05% Trition x-100, 20 mM Tris (pH: 8), 137 mM NaCl, 5% Glycerin, protease inhibitors (Sigma-Aldrich, Munich, Germany). Protein (10 µg per lane) were then separated by SDS-polyacrylamide gel electrophoresis and blotted onto PVDF (Amersham/GE Healthcare, Munich, Germany) membranes, saturated with 5% dried milk in PBS containing 0.05% Tween 20 (PBST) (Roth, Karlsruhe, Germany) over night. Blots were then incubated with the primary antibody: E-selectin (Santa Cruz, Heidelberg, Germany); P-selectin (abcam, Cambridge, UK); ICAM-1, (Biozol, Eching, Germany); VCAM-1 (Santa Cruz, Heidelberg, Germany); β-actin (BioCat, Heidelberg, Germany); primary antibody dilution 1:500–1:1000. Blots were then incubated with the appropriate second antibody; biotinylated goat anti-rat IgG, biotinylated goat anti-rabbit IgG or biotinylated horse anti-mouse IgG, (Linaris GmbH, Wertheim-Bettingen, Germany); secondary antibody dilution of 1:1000. Membranes were incubated with 1% dried milk in PBST and horseradish peroxidase streptavidin (Linaris GmbH, Wertheim-Bettingen, Germany). Bound antibodies were detected using an enhanced chemiluminescence substrate kit (Roentgen Bender, Baden Baden, Germany).
Immunofluorescence staining
CD3 staining was performed using goat anti-CD3e Ab (1:50; BD, Pharmingen, Heidelberg, Germany) and FITC anti-goat Ab (1: 100; BD, Pharmingen, Heidelberg). MPO staining was performed using a rabbit anti-MPO Ab (ready-made; NeoMarkers, Fremont, USA) and a donkey Cy3 anti-rabbit Ab (1:500; Dianova, Hamburg, Germany). For CD11c staining we used a rabbit FITC-CD11c Ab (1:30; BD, Pharmingen, Heidelberg).
Files in this Data Supplement:
- Figure S1. Enhanced CD11c+ cells in TNFR1−∕− ears (JPG, 194 KB) -
Ear sections from sensitized TNFR1−∕− and TNFR1+∕+ mice were fixed 24 h after ear challenge and then stained using (A) CD3 Ab (red), and MPO Ab (green); (B) CD11c Ab (green), Nuclear staining (Yopro; blue).
- Figure S2. Cy5 labeled mast cells in TNFR1−∕− ears (JPG, 172 KB) -
In vivo optical imaging investigation of directly (intra-cutaneously) into the right ear (of naïve TNFR1−∕− mice) injected Cy5 labeled mast cells. Mast cells were intra-cutaneously engrafted into the right ear 27 days prior in vivo investigation (mouse 1–2: 0.5 × 106 Cy 5 labeled mast cells; mouse 3: 0.5 × 106 red fluorescence protein (RFP) labeled mast cells; control).
- Figure S3. Reduced P-selectin, E-selectin, VCAM-1, and ICAM-1 protein expression in TNFR1−∕− ear tissue (JPG, 118 KB) -
Ear tissue from TNCB challenged TNFR1+∕+ (lanes 2 and 3) and TNFR1−∕− (lanes 4 and 5) mice, was harvested 4 h after TNCB challenge. P-selectin, E-selectin, ICAM-1, or VCAM-1 proteins were analyzed by western blot. Positive controls (++; lane 1) were from DTHR from a preexisting experiment with a slightly different sensitization protocol (5 consecutive TNCB challenges), to obtain a strong positive control.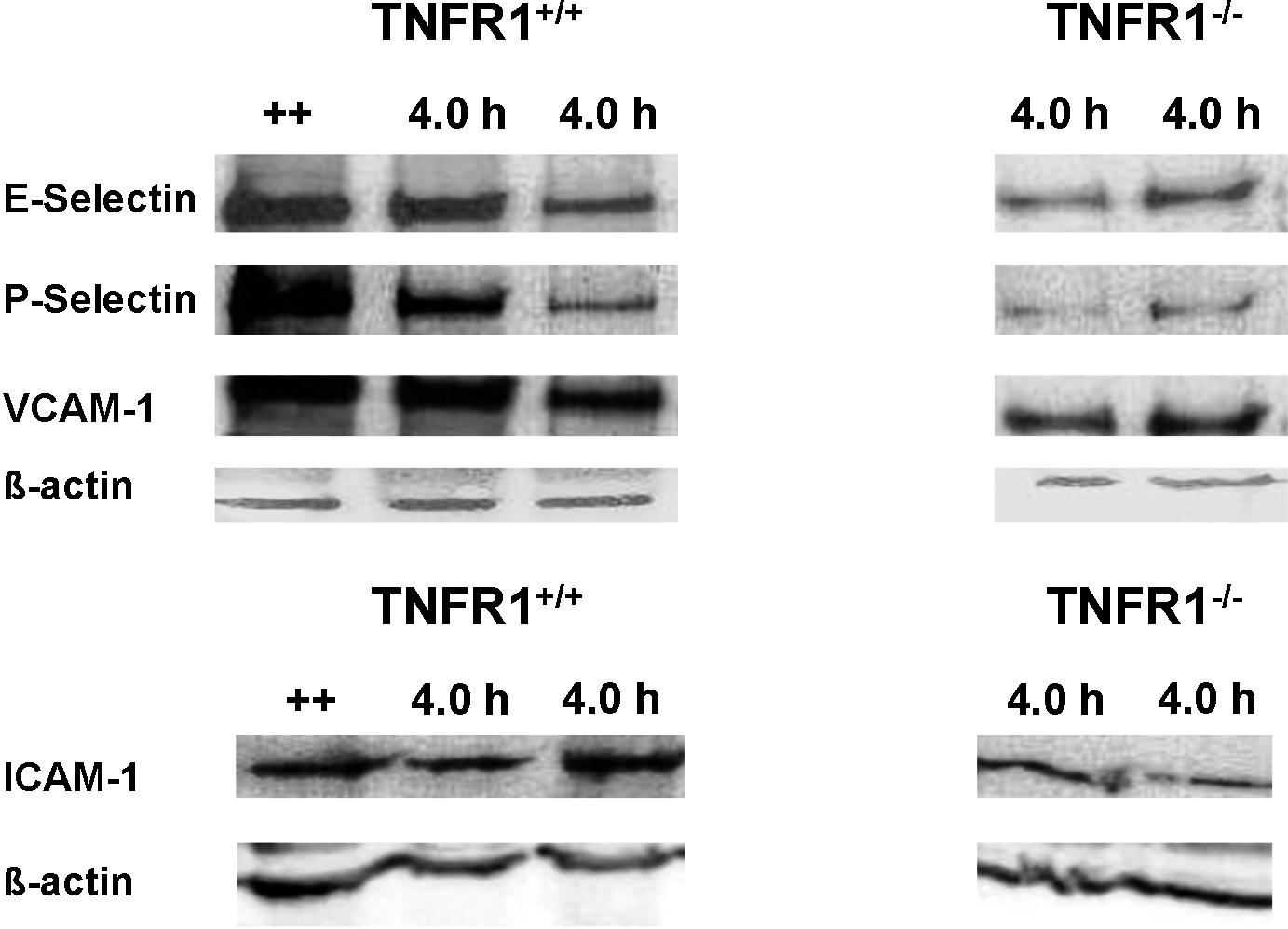
- Video 1. Reduced leukocyte adhesion in TNFR1−∕− mice (AVI, 5.83 MB) -
Non-invasive intravital microscopy images of leukocyte adhesion to vascular endothelia in TNFR1+∕+, 4.5 h after elicitation of DTHR. - Video 2. Reduced leukocyte adhesion in TNFR1−∕− mice (AVI, 10.3 MB) -
Non-invasive intravital microscopy images of leukocyte adhesion to vascular endothelia in TNFR1−∕− mice, 4.5 h after elicitation of DTHR.