Blood, Vol. 114, Issue 18, 3880-3889, October 29, 2009
Myeloma cell line–derived, pooled heat shock proteins as a universal vaccine for immunotherapy of multiple myeloma
Blood Qian et al. 114: 3880
Supplemental materials for: Qian et al
Files in this Data Supplement:
- Figure S1. Schematic presentation of mouse vaccination experiments (JPG, 277 KB) -
(A) Vaccinations as myeloma prophylaxis; (B) Vaccinations and other therapies as treatments for established myeloma.
- Figure S2. Protective effects of gp96 vaccines against myeloma development in tumor-B model (JPG, 217 KB) -
Balb/c mice (10 per group) were s.c. vaccinated twice with either gp96N, gp96A, gp96B, gp96C, gp96AC, or gp96ACD, followed by challenge with tumor-B cells. Tumor burdens were measured twice every wk. Mice were euthanized when s.c. tumors reached 225 mm2 or when mice became moribund. Results shown are measurements of tumor burdens and survival of mice received different treatments. *P P
- Figure S3. Protective effects of gp96 vaccines against myeloma development in tumor-D model (JPG, 226 KB) -
Balb/c mice (10 per group) were s.c. vaccinated twice with either gp96N, gp96A, gp96B, gp96D, gp96AB, or gp96ABC, followed by challenge with tumor-D cells. Tumor burdens were measured twice every wk. Mice were euthanized when s.c. tumors reached 225 mm2 or when mice became moribund. Results shown are measurements of tumor burdens and survival of mice that received different treatments. *P P
- Figure S4. Protective effects of gp96 vaccines against myeloma development in tumor-C model (JPG, 205 KB) -
Balb/c mice (10 per group) were s.c. vaccinated twice with either gp96N, gp96A, gp96B, gp96C, gp96AB, or gp96ABD followed by challenge with tumor-C cells. Tumor burdens were measured twice every wk. Mice were euthanized when s.c. tumors reached 225 mm2 or when mice became moribund. Results shown are measurements of tumor burdens and survival of mice that received different treatments. *P P
- Figure S5. Protective effects of gp96 vaccines against myeloma development in tumor E and F models (JPG, 172 KB) -
(A) C57BL/KaLwRij mice (5 per group) were s.c. vaccinated twice with either gp96N, gp96E, and gp96BCD, followed by i.v. challenge with tumor-E cells. (B) C3H mice (5 per group) were s.c. vaccinated twice with gp96N, gp96F and gp96BCD, followed by s.c. challenge with tumor-F cells. Tumor burdens were measured twice every wk. Mice were euthanized when s.c. tumors reached 225 mm2 or when mice became moribund. Results shown are measurements of tumor burdens and survival of mice that received different treatments. * P P
- Figure S6. gp96 vaccines induce CD4+ and CD8+ tumor-specific T-cell responses (JPG, 568 KB) -
Balb/c mice (3 per group) were s.c. vaccinated twice with either normal g96N, gp96B, gp96AC, gp96ACD, followed by challenge with tumor-B cells. One wk later, splenocytes were isolated, pooled, and restimulated with irradiated tumor-B cells for 5 d. Shown are the results of T cells from mice vaccinated with different gp96 vaccines: (A) T-cell proliferation measured by CFSE dilution assay on gated CD4+ and CD8+ T cells; (B) Intracellular staining for IFN-γ and IL-4 expression on gated CD4+ and CD8+ T cells; and (C) Cytotoxicity of CTLs against tumor-B cells. (D) Longevity of gp96 vaccine-induced immune responses. Balb/c mice were s.c. vaccinated twice with either normal g96N, gp96A, and gp96BCD. At mo 1, 2, and 3 from the second vaccination, mice (3 per group) were sacrificed and splenocytes were isolated, labeled with CFSE, and restimulated for 5 d with irradiated tumor A in vitro. T-cell proliferation was measured on gated CD8+ T cells by CFSE dilution. Shown are percentages of proliferating CD8+ T cells from mice vaccinated with either gp96N, gp96A, or gp96BCD before vaccination or 1, 2, or 3 mo after the second vaccination. Representative results of one out of two independent experiments are shown.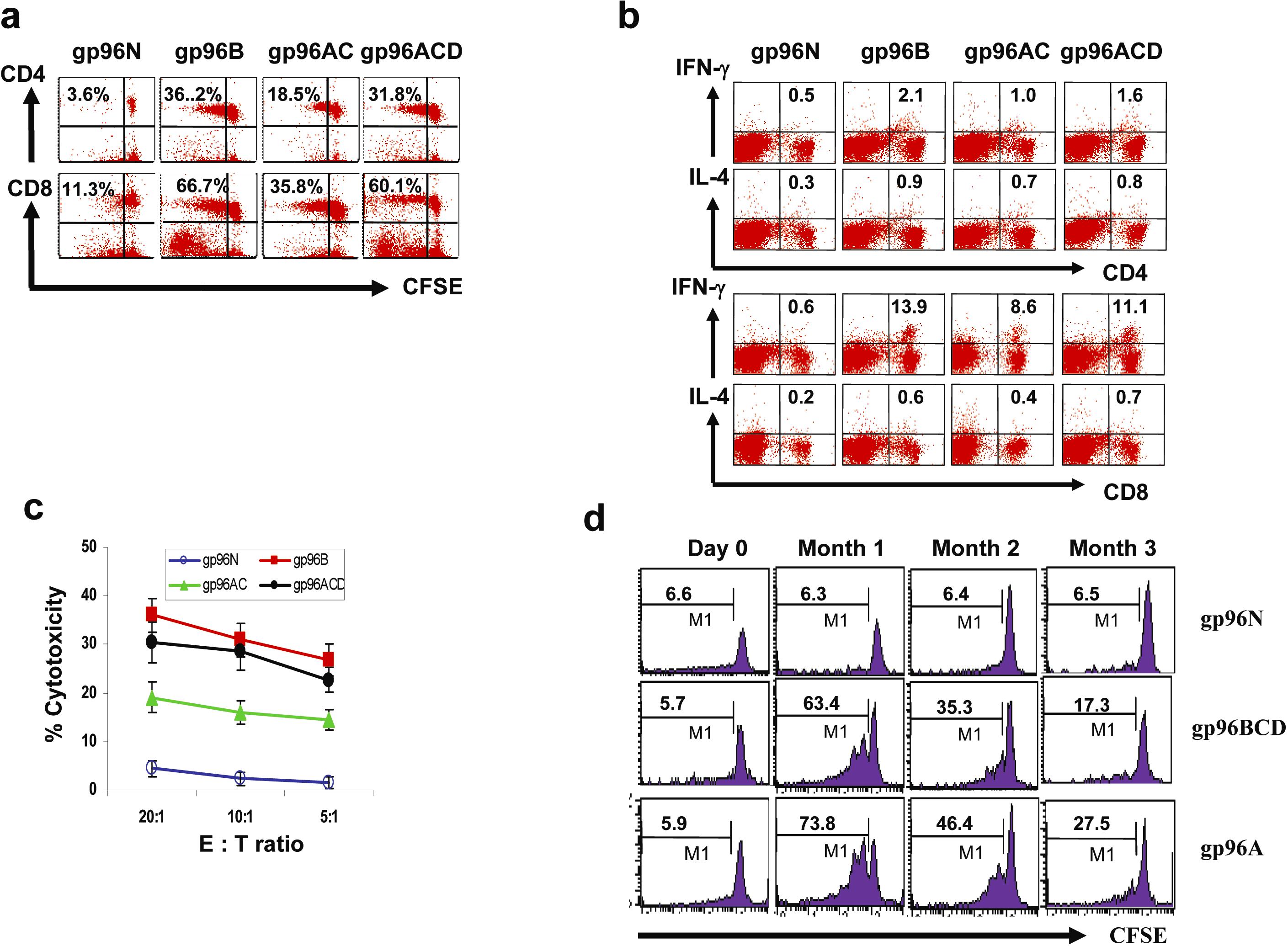
- Figure S7. Importance of T cells, NK cells, and IFN-γ in gp96 vaccine-induced tumor protection (JPG, 201 KB) -
Mice (5 per group) were s.c. vaccinated twice with pooled heterologous gp96ACD and then depleted of CD4+ or CD8+ T cells or NK cells by i.p. injections of specific mAbs before tumor-B challenge, or IFNγ−∕− mice were s.c. vaccinated twice with gp96ACD and followed by tumor-B challenge. Tumor burdens were measured twice every wk. Results shown are measurements of tumor burdens and survival of mice that received different treatments. * P P