Supplementary material for Nazif and Bogyo (2001) Proc. Natl. Acad. Sci. USA 98 (6), 2967–2972. (10.1073/pnas.061028898)
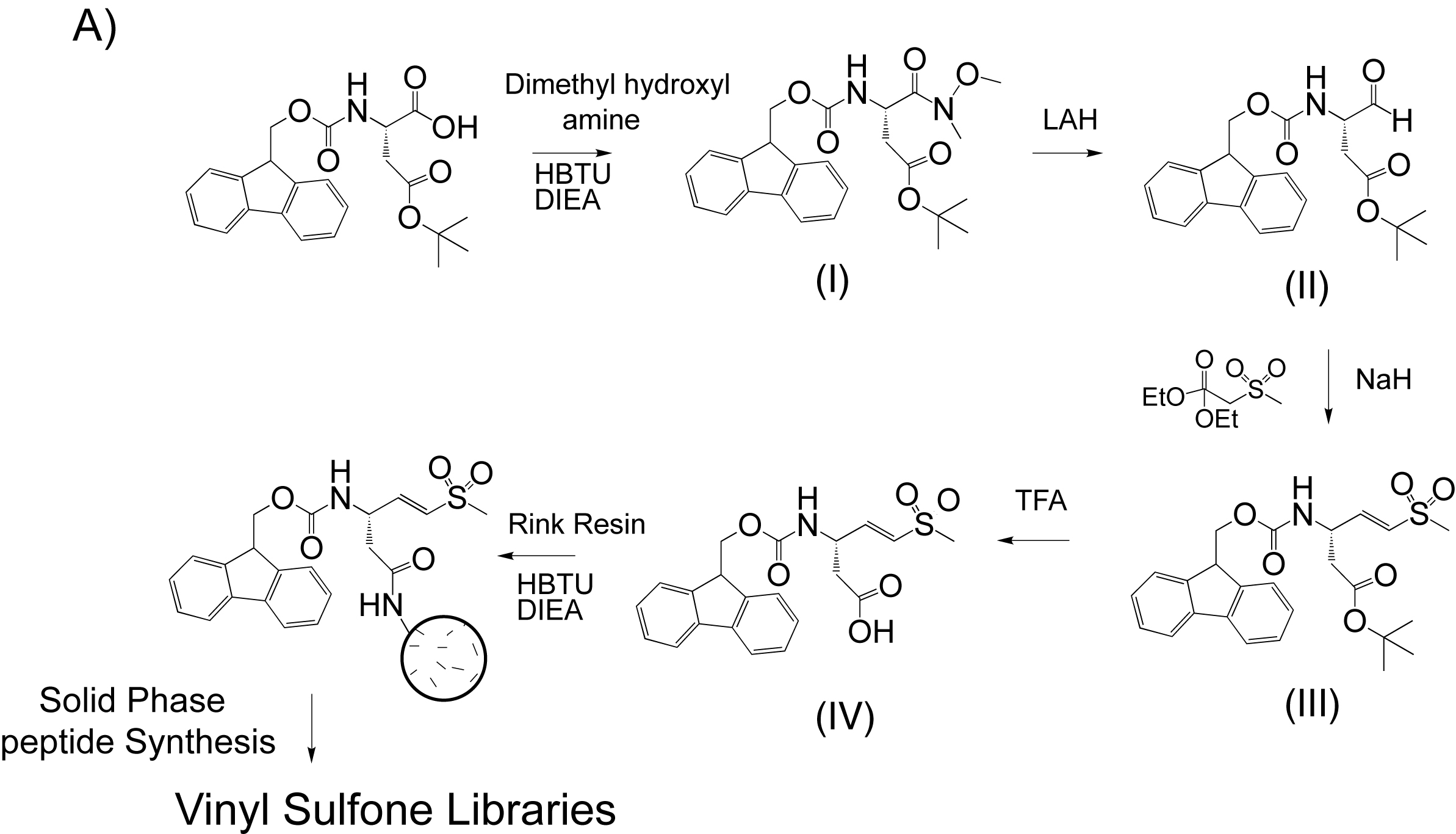
Synthetic Protocols
Synthesis of Fluorenylmethoxycarbonyl (Fmoc)-Asp(OtBu)-Dimethyl Hydroxyl Amide (I)
. Fmoc-Asp(OtBu)-OH (10 g, 24.3 mmol), hydroxybenzotriazole (3.6 g, 26.7 mmol), and dicyclohexylcarbodiimide (5.5 g, 26.7 mmol) were dissolved in dimethylformamide. After stirring 30 min at room temperature a solid formed and was removed by filtration. N,O-dimethyl hydroxyl amine (2.84 g, 29.2 mmol) was added as a solid to the filtered reaction mixture along with triethyl amine (2.94 g, 29.2 mmol). The reaction was stirred for an additional 12 h and then concentrated by rotary evaporation of the solvent. The crude oil then was dissolved in ethyl acetate and extracted with three portions each of saturated sodium bicarbonate, 0.1 M hydrochloric acid (HCl), and brine. The organic layer was dried over magnesium sulfate and concentrated to dryness. This crude preparation was used in subsequent reactions without further purification.Synthesis of Fmoc-Asp(OtBu)-H (II)
. Crude I (11.14 g, 24.5 mmol) was dissolved in anhydrous ethyl ether and stirred on ice under a positive argon flow. Lithium aluminum hydride (0.928 g, 24.5 mmol) was slowly added, leading to gas evolution. The reaction was stirred for an additional 20 min and then quenched by the addition of potassium hydrogen sulfate (6.67 g, 49 mmol). The quenched reaction was stirred on ice for 20 min and then at room temperature for an additional 30 min. The mixture was extracted three times with ethyl acetate, and the combined organic layers were washed with three portions of 0.1M HCl, saturated sodium carbonate, and brine. The organic phase was concentrated to an oil. The product was purified by flash chromotography in hexane/ethyl acetate (2:1, vol/vol). Yield was 5.58 g, 58.3%.Synthesis of Fmoc-Asp(OtBu)-Vinyl Sulfone (III)
. Diethyl (methylthiomethyl) phosphonate was oxidized to the corresponding phosphonate sulfone using peracetic acid in aqueous dioxane as described (1). Diethyl phosphonate sulfone (4.06 g, 17.66 mmol) was dissolved in anhydrous tetrahydrofuran (THF) under argon. Sodium hydride (678 mg, 16.96 mmol) was added and the reaction was stirred for 30 min at room temperature. Pure II (5.58 g, 14.1 mmol) was dissolved in anhydrous THF under argon and added to the stirring reaction by cannula. The reaction was then allowed to stir for 30 min and was quenched with water, and the resulting aqueous phase was washed three times with dichloromethane. The organic phases were combined, dried over anhydrous magnesium sulfate (MgSO4), and evaporated to dryness. The crude oil was purified by flash chromatography over silica gel in hexane/ethyl acetate (1.5:1 vol/vol). Yield was 4.1 g, 8.7 mmol, 62%.Synthesis of Fmoc-Asp-Vinyl Sulfone (IV)
. Pure III (4.1 g, 8.7 mmol) was dissolved in methylene chloride (10 ml) and an equal volume of anhydrous trifluoroacteic acid was added. The reaction was stirred for 4 h and quenched by the addition of an excess volume of toluene. The reaction was concentrated by rotary evaporation and then resuspended in toulene. The reaction mixture was evaporated to an oil and the product was precipitated by the addition of ethyl ether. The resulting white precipitate was collected by centrifugation dried under vacuum. Yield was 2.95 g, 81%.Coupling of Fmoc-Asp-Vinyl Sulfone (IV) to Rink Amide Resin
. The pure product IV was coupled to Rink amide resin (0.8 mmol/g resin load) in dimethylformamide using standard coupling chemistry (diisoproplycarbodimide, hydroxybenzotriazole), and resin load was determined by absorbance of free Fmoc upon deprotection. The resin was dried under vacuum and used as the starting material for the synthesis of all compound libraries.Reference
1. Bogyo, M., Shin, S., McMaster, J. S. & Ploegh, H. (1998) Chem. Biol. 5, 307–320.