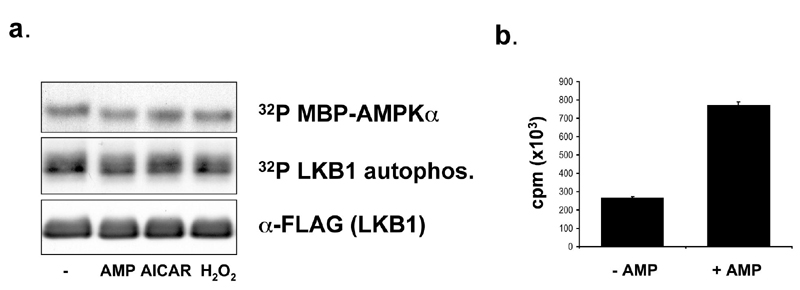
Fig. 8.
LKB1 kinase activity is not increased by AMP in vitro, nor by stimulation of cells with known AMP-activated protein kinase (AMPK) activators. (a) FLAG-tagged LKB1 immunoprecipitated from mammalian cells as described in Fig. 1 was assayed for the ability to phosphorylate the maltose-binding protein (MBP)–AMPK (1-312) fusion in response to 5-aminoimidizole-4-carboxamide riboside (AICAR) or H2O2 treatment of cells followed by an LKB1 immunoprecipitate kinase assay, or from untreated cells with and without 300 m M AMP in the in vitro kinase assay as previously described (1). (b) Parallel control kinase reaction of purified rat liver AMPK (Upstate Biotechnology, Lake Placid, NY) demonstrates stimulation of AMPK in vitro kinase activity toward SAMS peptide by 300 m M AMP in the kinase reaction buffer as above. autophosph., autophosphorylation.1. Davies, S. P., Carling, D. & Hardie, D. G. (1989) Eur. J. Biochem. 186, 123-128.