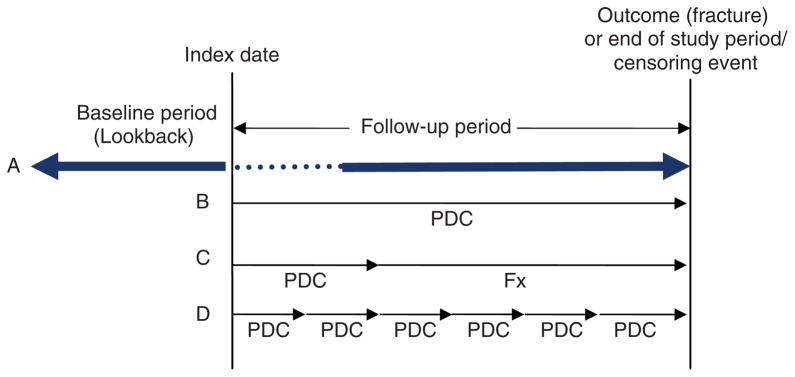
Figure 1.
Depiction of a database cohort study design to measure compliance and impact on fracture risk. Index date is identified by the prescription date that defines cohort entry. (A) The baseline (lookback) period is the period of interest prior to the first prescription (index date), and study follow up occurs after the index date and until the outcome or censoring date. Fracture outcomes can be assessed either throughout the entire follow-up period or after a ‘treatment onset’ (dotted line) period. The three primary methods to measure compliance include use of: (B) the entire follow-up period, (C) an ascertainment period, and (D) a time-varying measure. PDC, proportion of days covered (measure of compliance) = total days of drug supplied in the observation period divided by the total days in the observation period and capped at 100%; Fx = specific fracture follow-up period.