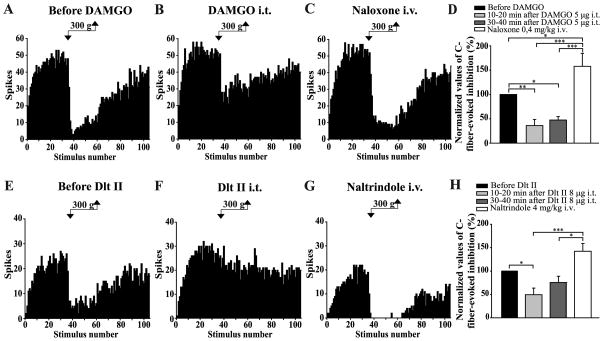
Figure 4. Intrathecal DAMGO and Dlt II both inhibit the DNIC triggered by a noxious mechanical stimulus.
(A–C and E–G) Histograms showing representative C-fiber-evoked responses of 2 trigeminal WDR neurons to 105 successive electrical stimulations recorded before (A, E) and 30 min after intrathecal (i.t.) administration of DAMGO 5 μg (B) or 10 min after Dlt II 8 μg (F). Between the 36th and 60th stimulation, a 300 g mechanical pressure was applied on one hindpaw of the animal with calibrated forceps. The DNIC triggered by mechanical noxious stimulation of the hindpaw are reduced after both DAMGO (B) and Dlt II (F) injection. The intravenous (i.v.) administration of naloxone (C) and naltrindole (G) reversed the effect of DAMGO and Dlt II, respectively. (D, H) Graphic representation showing the mean (n=8 for D, and n=9 for H) percentage of inhibition of C-fiber-evoked action potentials before, 10–20 minutes and 30–40 minutes after the intrathecal injection of the opioids. Note that the data are individually normalized to those before administration of the drugs. The selective-MOPR agonist significantly reduced the percentage of inhibition of C-fiber-evoked action potentials either 10–20 or 30–40 minutes after its administration. Naloxone significantly reversed those opioidergic-induced effects (D). The selective-DOPR agonist significantly reduced the percentage of inhibition of C-fiber-evoked action potentials only 10–20 minutes after its administration and naltrindole significantly reversed this DOPR-mediated effect (D). *p<0.05, **p < 0.01 and ***p < 0.001, one-way ANOVA for repeated measures with Bonferroni’s post hoc test. Error bars indicate the Standard Error of the Mean (SEM).