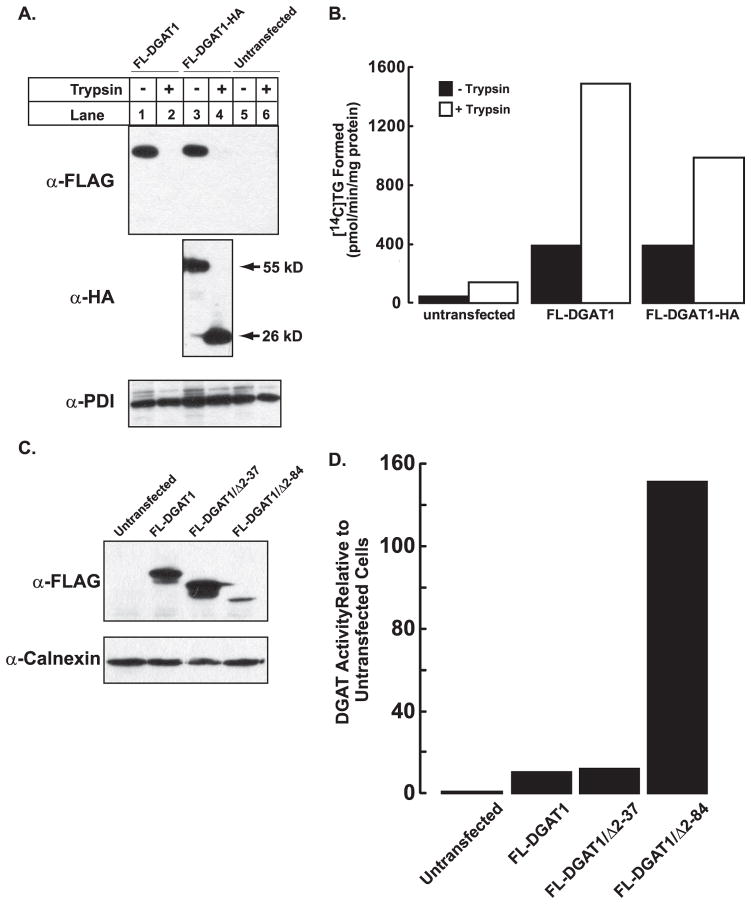
FIGURE 8. Stimulation of DGAT1 in vitro activity in the absence of its N terminus.
Total membranes from untransfected HEK-293T cells and HEK-293T cells expressing FL-DGAT1 and FL-DGAT-HA were prepared as described under “Experimental Procedures.” Aliquots of membranes (50 μg protein) were incubated in the absence or presence of20 μg/ml of trypsin (as described in Fig. 3). After the addition of soybean trypsin inhibitor, samples were immunoblotted with anti-FLAG, anti-HA, and anti-PDI antibodies (A), and in vitro DGAT activity was determined (B). C, an immunoblot shows transient expression of FL-DGAT1, DGAT1/Δ2–37, and FL-DGAT1/Δ2– 84 in HEK-293T cells. Total cellular membranes (50 μg of protein) were immunoblotted with anti-FLAG. D, shown is in vitro DGAT activity in membranes from cells expressing FL-DGAT1, DGAT1/Δ2–37, and FL-DGAT1/Δ2– 84. Membranes from cells were assayed for in vitro DGAT activity as described in Fig. 2. The activities of the various mutants were normalized to the amount of FL-DGAT1 proteins that were quantified using a fluorescently labeled secondary antibody. The fluorescence signal was obtained with a VersaDoc 4000 imaging system (Bio-Rad) and quantified with Quantity One imaging software. The activities for untransfected cells and cells transfected with FL-DGAT1 were 64.2 and 690.2 pmol/min/mg of protein, respectively. Data are from one experiment, performed in duplicate, which was repeated once with similar results.