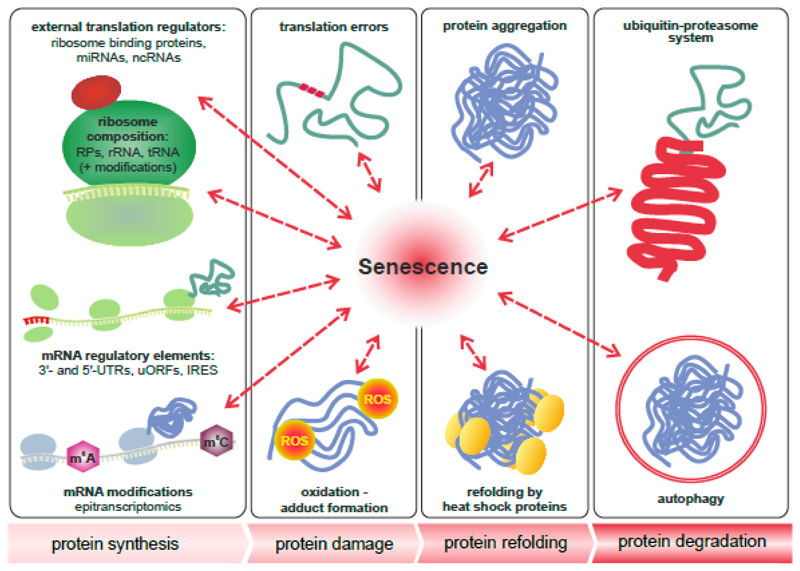
Figure 4. The role of senescence in multi-level proteostasis - the four stages in the life of a protein.
Cellular senescence and aging presumably influence all four stages in the life of a protein, namely its synthesis by ribosomes, the accumulation of damage, refolding and aggregation, as well as its degradation. Protein synthesis is tightly controlled by the composition of ribosomes, association of the core translation machinery with external regulators, mRNA modifications and specific sequence elements of mRNAs, such as the 3’ and 5’ untranslated regions (UTRs), short upstream open reading frames (uORFs) or internal ribosome entry sites (IRES). Translation errors and oxidation lead to damaged and misfolded proteins, which are prone to aggregation. This process is counteracted by heat shock proteins or molecular chaperones. Damaged and aggregated proteins are degraded by autophagy or via the ubiquitin-proteasome system. Both refolding and degradation are impaired in cellular and organismal aging and are tightly interconnected with other stages of proteostasis.