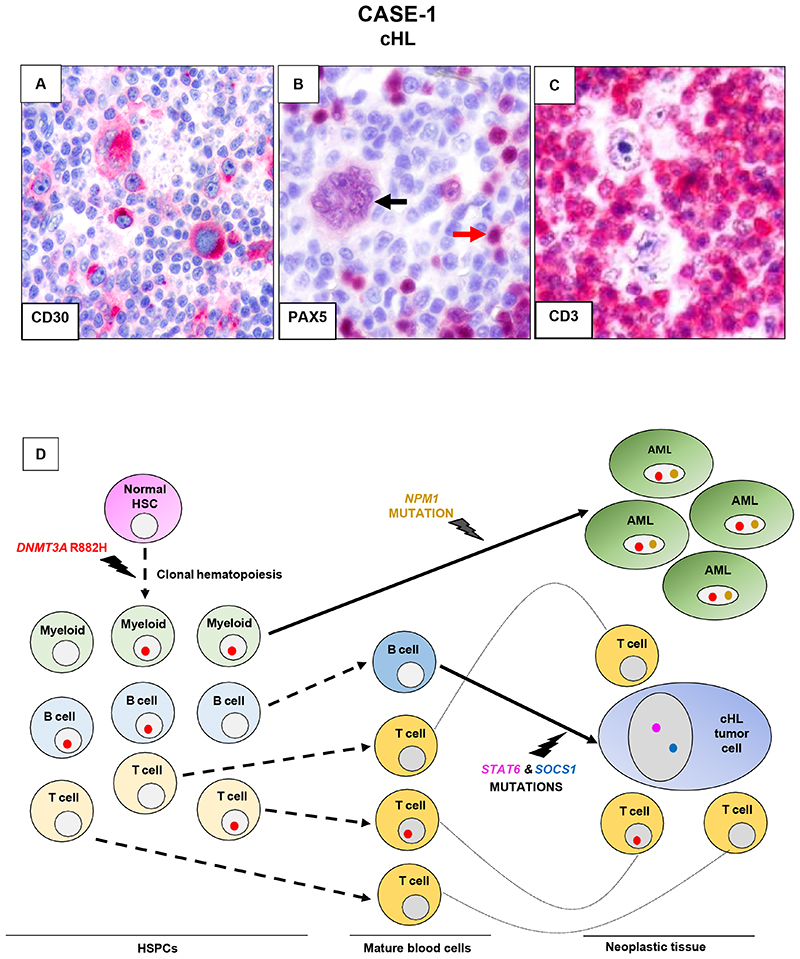
Figure 2. Trajectories of clonal hematopoiesis in a patient sequentially developing cHL and AML (case 1).
A-C. Nodular sclerosis cHL lymph node biopsy immunostained (red labeling) for CD30 shows various HRS cells strongly expressing this cHL diagnostic marker (A). Immunostaining for the B cell-specific transcription factor PAX5 (B) shows nuclear positivity in a multinucleated Reed-Sternberg (black arrow) and, to a higher intensity, in sparse bystander B cells (one indicated by the red arrow). In C, CD3 immunostaining highlights the abundant reactive T cells in the microenvironment surrounding HRS cells.
D. Spreading of the DNMT3A R882Hmutation: i) through preneoplastic hematopoiesis in the bone marrow and blood, with multilineage myeloid, B-cell and T-cell involvement and then ii) through the neoplastic tissues of AML and cHL, in the latter involving reactive tissue cells (mostly T cells) but not HRS cells. Also indicated are the genetic lesions leading to the development of the AML clone (NPM1 mutation) and the HRS cell clone (STAT6 and SOCS1 mutations), arising respectively from a myeloid progenitor belonging to clonal hematopoiesis and a mature B cell not belonging to clonal hematopoiesis. HSC, hematopoietic stem cell.