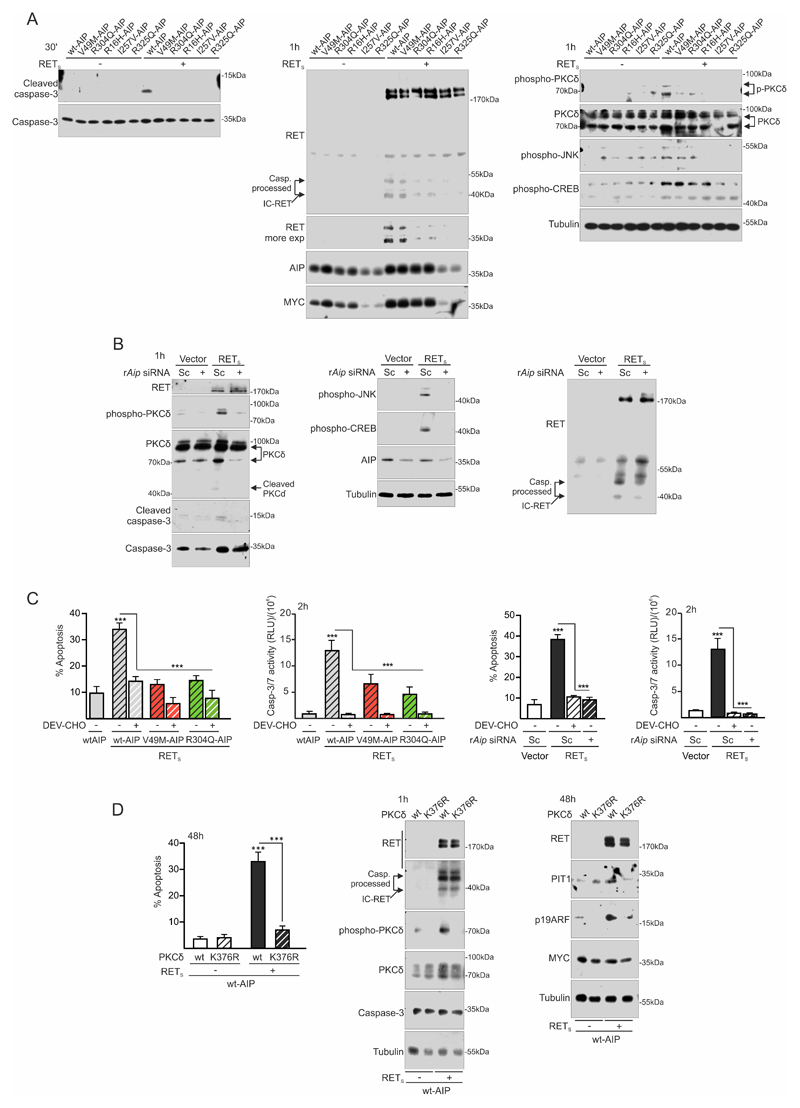
Figure 3. AIP variant or Aip repression alter the RET-apoptotic pathway at an early step: inhibition of caspase-3 and PKCδ activation.
A) Plasma membrane fractioning extracts 30min after low serum conditions show that variant AIP block early caspase-3 cleavage and activation. At 1 h, intracellular IC-RET processing is downregulated in the presence of variant AIP. p-PKCδ is concordantly downregulated. p-JNK is also blocked. This time is too early to see any consistent change in p-CREB, previously described after 24 hours of GDNF deprivation. B) rAip siRNA blocks caspase-3 cleavage and activation together with p-PKCδ induction. In this case, p-JNK and p-CREB increases are also blocked by rAip siRNA, indicating different time-course than with variant AIP transfection. Caspase-3 processed IC-RET bands are downregulated in the presence of rAip siRNA. C) Quantification of caspase activity shows that caspase-3 activation is an early event after GDNF deprivation that can be blocked by the caspase-3 peptide inhibitor DEV-CHO or variant AIP or rAip siRNA in a similar fashion. This corresponds well with the later detected apoptosis. D) Apoptosis is absolutely dependent on PKCδ activity since the kinase-dead mutant K376R PKCδ blocks RET with wtAIP dependent apoptosis. Kinase-dead PKCδ does not alter IC-RET processing by caspase-3.
(Two-ways ANOVA with Sidak’s multiple comparison test correction C-D. ***, p<0.01)