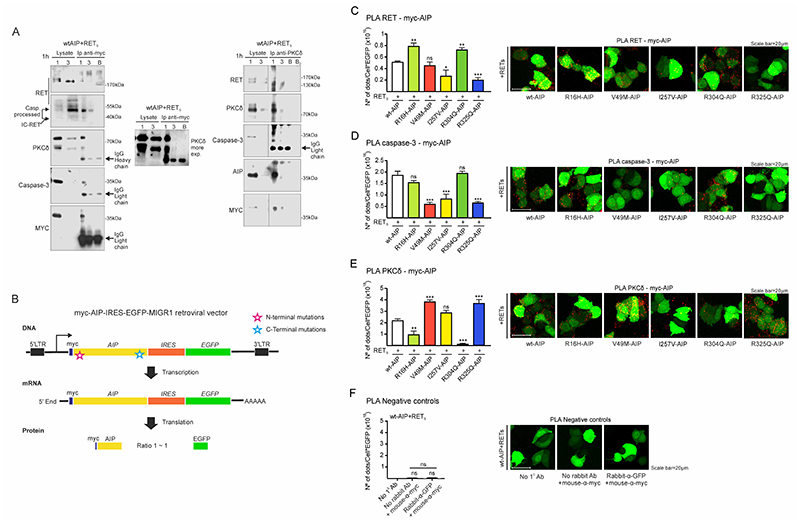
Figure 4. Molecular interactions between AIP, RET, PKCδ and caspase-3 are altered by either N-term or C-term AIP point mutations.
A) wtAIP (Myc-tagged) binds with RET, PKCδ and caspase-3 at the cytoplasm as shown by anti-myc immunoprecipitation of plasma membrane/cytoplasm extracts (1) and nuclear extracts (3), obtained 1 hour after serum deprivation. As control, a blank (B) immunoprecipitation with antibody and beads without cell extracts. Left: RET, IC-RET, caspase-3, PKCδ and AIP are co-immunoprecipitated with anti-Myc-tag antibody. Right: RET, caspase-3, AIP and PKCδ are co-immunoprecipitated with PKCδ antibody. In both types of immunoprecipitations, a very slight band of PKCδ or Myc-Aip can be detected in the nuclear fraction. B) Cartoon showing the constructs designed for the quantitative study of the interaction of wtAIP or each AIP variant with RET, caspase-3 and PKCδ through PLA assay. Each construct expressed similar amounts of AIP and EGFP, detected with GFP antibody and used as reference value in each cell. C) PLA assay between RET and AIP (myc-AIP). I257V and C-terminal R325Q variant significantly reduces the interaction: representative confocal microphotographs and quantification (n=6 per AIP variant). D) PLA assay between caspase-3 and AIP. Both N-terminal V49M and C-terminal R325Q variants, together with I257V, significantly reduce the interaction. E) PLA assay between PKCδ and AIP. Both N-term R16H and R304A variants significantly reduce the interaction, while V49M and R325Q enhanced the interaction, these are the residues that interact with caspase-3. I257V does not alter the interaction.
(One-way ANOVA with Holm-Sidak’s multiple comparison test correction C; Kruskal-Wallis with Holm-Sidak’s D, Dunn’s multiple comparison test correction E-F. **, p<0.01; ***, p<0.001; ns, non-significant)