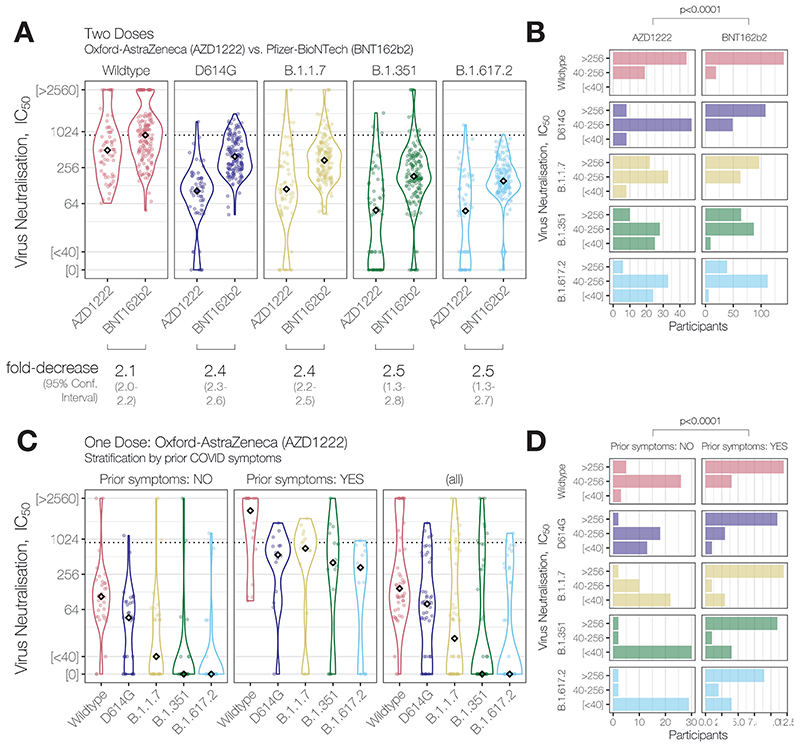
Figure 1. Neutralising antibody activity against SARS-CoV-2 variants of concern B.1.617.2 and B.1.351 elicited by partial or full vaccination with ChAdOx1 nCoV-19 (AZD1222, Oxford-Astra-Zeneca) and effect of reported prior COVID symptoms.
(A) Neutralising antibody titres (NAbTs) against five SARS-CoV-2 strains from 63 study participants who had received 2 doses of ChAdOx1, comparised to 159 participants who had received 2 doses of BNT162b2. NAbTs are expressed as serum fold-dilution required to achieve 50% virus neutralisation (IC50), and shown (B) grouped into 3 response levels. (C) NAbTs from 50 participants following 1 dose of AZD1222, stratified according to participants’ report of prior COVID symptoms, and (D) grouped into 3 response levels.