Abstract
Soft-bodied slow moving sea creatures such as sea stars and sea cucumbers lack an adaptive immune system, and have instead evolved the ability to make specialized protective chemicals (glycosylated steroids and triterpenes) as part of their innate immune system. This raises the intriguing question of how these biosynthetic pathways have evolved. Sea star saponins are steroidal, while those of sea cucumber are triterpenoid. Sterol biosynthesis in animals involves cyclization of 2,3-oxidosqualene to lanosterol by the oxidosqualene cyclase (OSC) enzyme lanosterol synthase (LSS). Here we show that sea cucumbers lack LSS and instead have two divergent OSCs that produce triterpene saponins, and that are likely to have evolved from an ancestral LSS by gene duplication and neo-functionalization. We further show that sea cucumbers make alternate sterols that confer protection against self-poisoning by their own saponins. Collectively these events have enabled sea cucumbers to evolve the ability to produce saponins and saponin-resistant sterols concomitantly.
Introduction
Echinoderms lack adaptive immunity and so rely exclusively on their innate immunity for growth and survival in the hostile benthic environment1. As part of this innate immunity, soft-bodied slow-moving echinoderms such as sea cucumbers and sea stars produce specialized metabolites (glycosylated steroids and triterpenes, also known as saponins) that provide chemical defence against potential assailants2–3. In contrast, the closely related sea urchins are protected by spines and do not make such compounds4 (Fig. 1a). When stressed, as a first line of defence, some sea cucumbers expel sticky threads called Cuvierian tubules which entangle their enemies and immobilize them6. Immobilized victims eventually die due to the saponins of these tubules6. In addition to defense, saponins also have other biological functions in sea creatures, including in reproduction, spawning, and chemical communication with symbionts7–10. Many animals use toxins as chemical defences. These protective compounds are usually either sequestered from food or produced by endosymbionts. In contrast, echinoderms biosynthesise their toxins themselves1. Saponins have antifungal activity that is attributed to their ability to form complexes with membrane sterols such as cholesterol (5), so causing membrane permeabilization and cell death. Sea stars and sea cucumbers make alternate, unusual sterols (lathosterol, 7 and 14α-methyl cholest-9(11)-en-3β-ol, 11) that protect against potential self-poisoning by endogenous saponins (Fig. 1b)11. Sea cucumbers are a food delicacy in South Asia and their extracts (of which saponins are important bioactive components) are highly valued for their medicinal properties. For these reasons, sea cucumber cultivation is a multimillion-dollar industry12. Despite the biological and commercial importance of sea cucumber saponins, the biosynthetic pathways for these compounds and how they have evolved in marine animals are unknown.
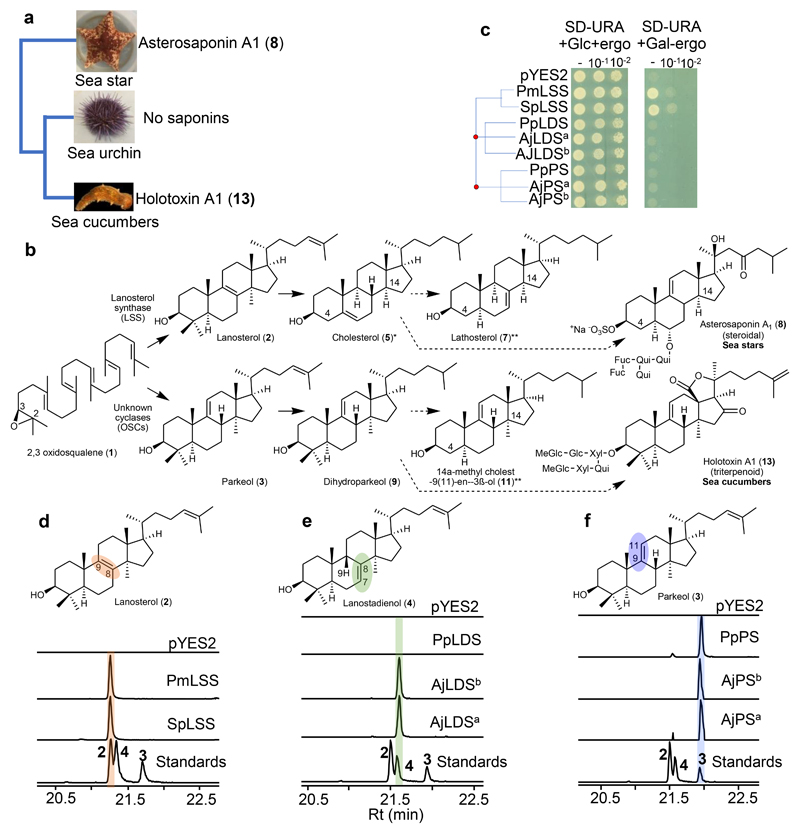
Fig. 1. Evolution of divergent OSCs in sea cucumbers.
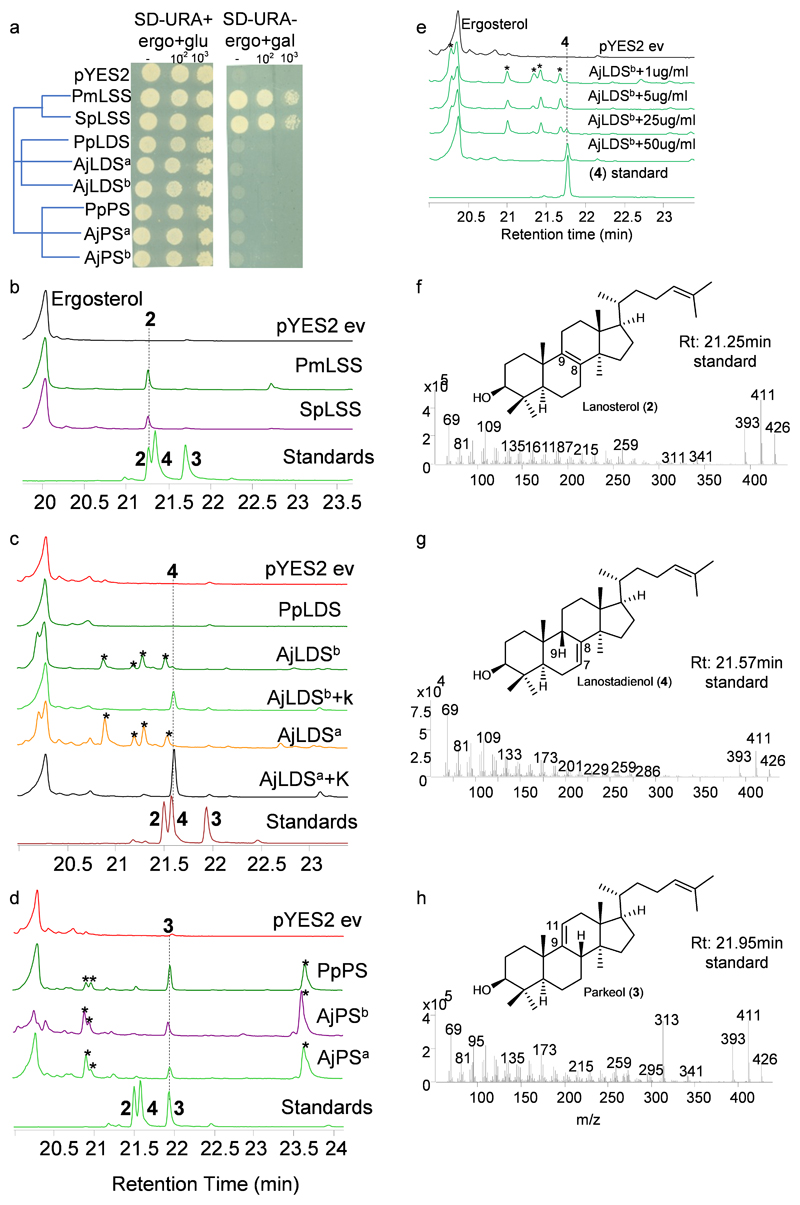
a, Presence/absence of saponin chemical defenses in slow moving, soft-bodied echinoderms. Tree drawn as in Ref.17. b, Biosynthetic origin of steroidal and triterpene saponins, and usual/unusual sterols, in sea stars and sea cucumbers. *, usual sterol, characterized by a common C5 unsaturation as well as absence of methyl groups at carbon positions 4 and 14; **, unusual sterols with C7 and C9(11) unsaturation. Solid and dotted arrows represent single and multiple steps, respectively. c, Complementation of the LSS-deficient yeast strain Gil77 with cloned OSCs. pYES2, empty vector control. From stock cultures yeast was spotted undiluted (-), and diluted 10 and 100-fold. d-f, GC-MS profile of yeast extracts expressing clade I OSC candidates (d), clade II OSC1 (e), and OSC2 candidates (f). In the LDS experiments ketoconazole (50 μg/ml) was included in the medium to limit in vivo modifications of OSC1 products by the endogenous yeast CYP51 enzyme (Extended Data Fig. 2c, Methods). GC-MS peaks in d-f are extracted ion chromatograms (EIC) for the ion m/z 426. The corresponding total ion chromatograms (TIC) and mass spectra are shown in Extended Data Fig. 2b-h. The lower chromatograms in d-f show GC traces for an equimolar mixture of the standards lanosterol (2), lanostadienol (4) and parkeol (3). Pm, sea star Patiria miniata; Sp, sea urchin Strongylocentrotus purpuratus; Aj, sea cucumber Apostichopus japonicus; Pp, sea cucumber Parastichopus parvimensis; Sc, sea cucumber Stichopus chloronotus. Superscripts ‘a’ and ‘b’ for AjLDS and AjPS denote different accessions of A. japonicus. Rt; retention time.
Sea star saponins are steroidal (e.g. asterosaponin A1, 8), while those made by sea cucumbers are triterpenoid (e.g. holotoxin A1, 13), the major difference being the presence/absence of methyl groups at carbon positions C4 and C14 (Fig. 1b, Supplementary Fig. 1). Both types of compounds originate from the linear precursor 2,3-oxidosqualene (1)13 (Fig. 1b). In animals the first committed step in sterol biosynthesis involves cyclization of 2,3-oxidosqualene (1) to lanosterol (2) by oxidosqualene cyclase (OSC) enzymes known as lanosterol synthases (LSSs) (Fig. 1b). Lanosterol (2) is subsequently converted to the essential sterol cholesterol (5), which also serves as a precursor for steroidal saponins in sea stars. Triterpenoid saponins are widespread in plants but rare in animals, sea cucumbers being a noteworthy exception. In plants, the OSC gene family has expanded and diversified to produce an array of diverse triterpene scaffolds, with an average of 10-15 OSC genes per diploid plant genome13. In contrast, animals normally have a single OSC gene that encodes the LSS required for the synthesis of essential sterols13. The enzymes for the biosynthesis of steroids and triterpenoids and of saponins derived from these scaffolds in marine animals are not known (Fig. 1b). Here we take a genome mining approach to investigate the origins of steroids and triterpenoids in marine animals. We identify candidate OSC genes from sea stars, sea urchins and sea cucumbers, functionally characterize these by heterologous expression in yeast, and determine their product specificities (for sterol precursors or triterpene scaffolds). We further investigate the likely biological roles of two divergent non-LSS OSCs in sea cucumber. Our study provides the first insights into the emergence of triterpene biosynthesis in the sea cucumber lineage and the co-evolution of this with the ability to produce unusual sterols that are saponin-resistant and so are likely to provide protection against self-poisoning.
Results
Discovery and functional analysis of echinoderm OSCs
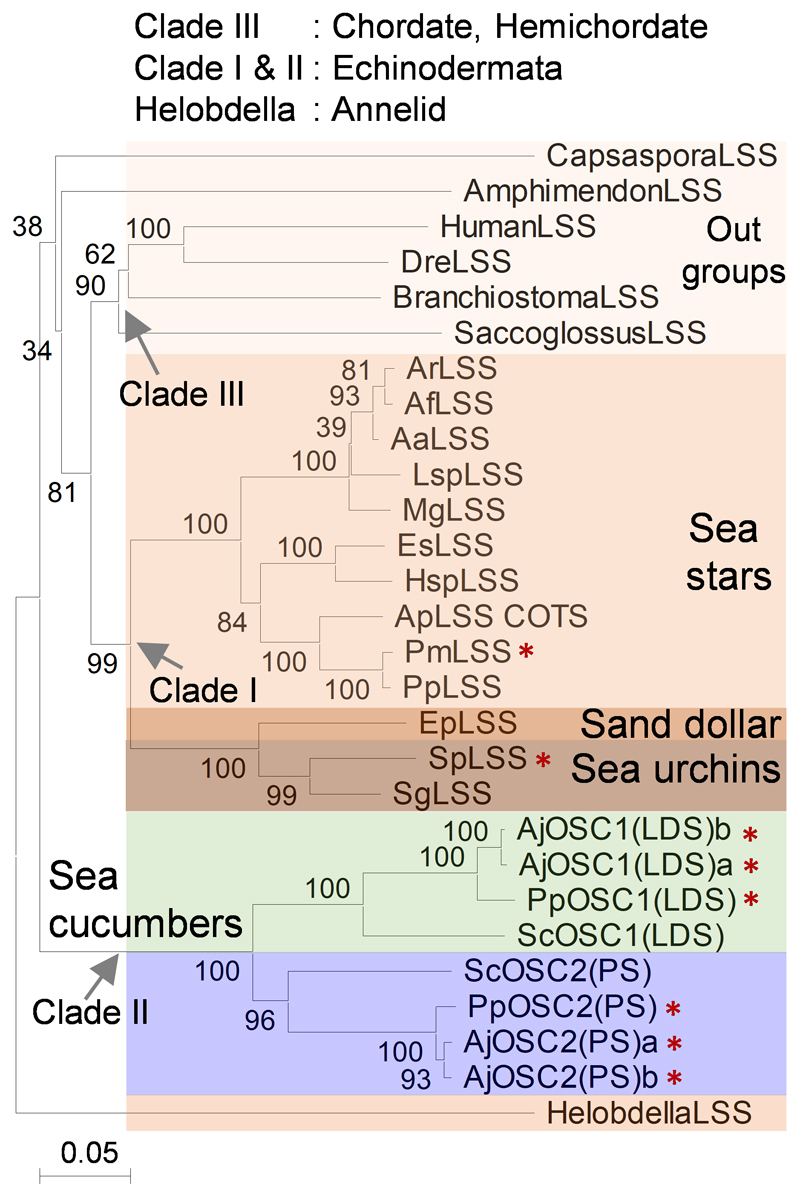
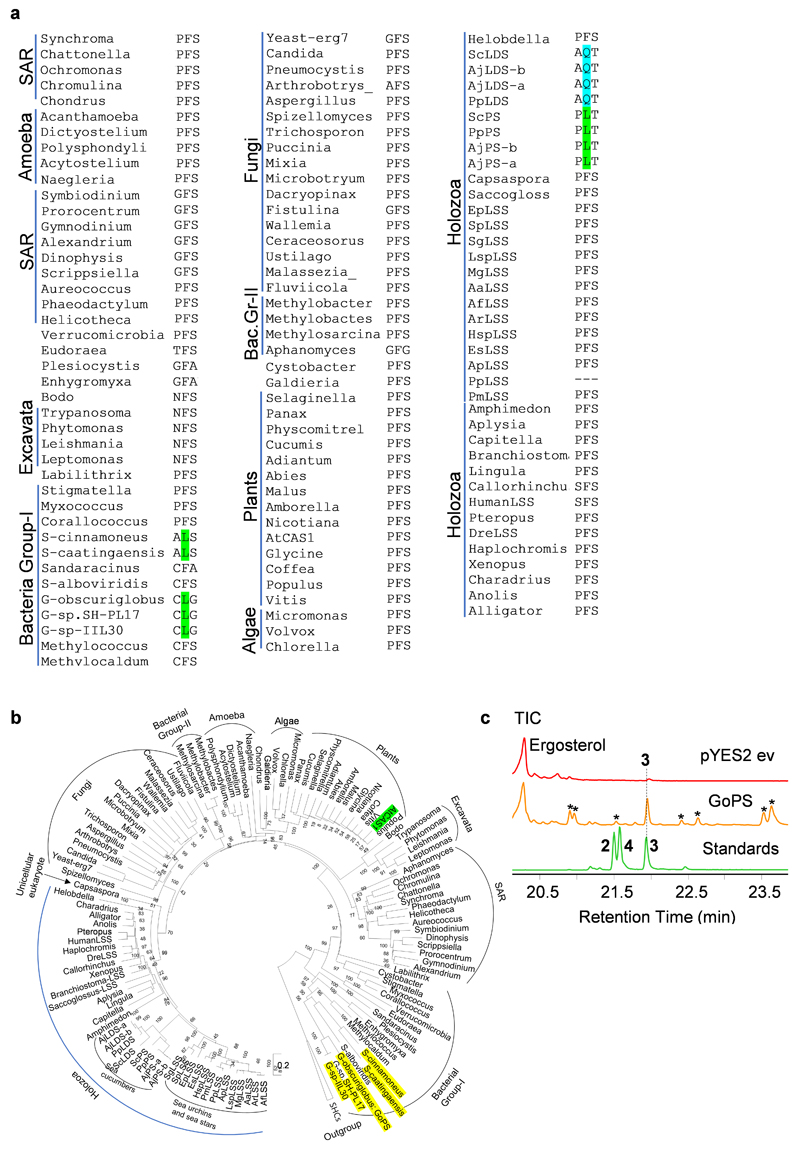
A sea cucumber genome sequence has recently been released14. To investigate the occurrence and types of OSCs in echinoderms and the Holozoan lineage more broadly, we mined the sequenced genomes of sea stars15,16, sea urchins16 and sea cucumbers14,17 for predicted OSC genes using human LSS as a template (Supplementary Table 1). Phylogenetic analysis reveals two distinct clades within the Echinodermata: OSCs from sea stars and sea urchins group together in clade I, while those from sea cucumbers form a distinct cluster, which we have named clade II (Extended Data Fig. 1). The differentiation of these latter OSCs from those in clade I is suggestive of functional divergence (Extended Data Fig. 1). Two OSC genes were recovered from each of three different sea cucumber species. Sea cucumbers are unusual in having two OSC genes, all other animals having just one (Supplementary Table 2).
The genes for the OSCs marked with red asterisks in Extended Data Fig. 1 were cloned and their functions determined by expression in the LSS-deficient yeast strain Gil7718. This enabled us to evaluate the OSCs for their ability to complement LSS deficiency in vivo, and also to investigate the nature of the 2,3-oxidosqualene (1) cyclization products generated by GC-MS analysis of yeast cell extracts. The OSCs were expressed under the control of the galactose responsive GAL1 promoter, which is repressed in the presence of glucose. In yeast, lanosterol (2) is the precursor for the biosynthesis of the essential sterol, ergosterol18. In the presence of exogenously supplied ergosterol and glucose, all of the yeast strains containing the different OSC constructs grew (Fig. 1c, Extended Data Fig. 2a). In the presence of galactose and the absence of exogenous ergosterol, two of the OSCs tested (sea star PmLSS and sea urchin SpLSS) complemented the growth of Gil77, suggesting that they are functional LSSs (Fig. 1c). GC-MS analysis confirmed the presence of lanosterol (2) in extracts from the strains expressing these two OSCs (Fig. 1d, Extended Data Fig. 2b, f).
None of the OSCs from sea cucumber restored Gil77 growth in the absence of ergosterol (Fig. 1c, Extended Data Fig. 2a). This may be because either they were not expressed in functional form, or alternatively because they make products other than lanosterol (2). GC-MS analysis of yeast extracts (cultured with ergosterol and galactose supplementation) revealed that two OSCs from the Apostichopus japonicus sea cucumber accessions (AjLDSa and AjLDSb) both yielded a new peak with a retention time of 21.6 min (Fig. 1e, Extended Data Fig. 2c, e, g). Large-scale yeast expression, purification and NMR characterization showed this to be 9β-lanosta-7, 24 dienol (4), a very closely related isomer of lanosterol (2) with a double bond at the Δ7(8) carbon position as opposed to the Δ8(9) position (Supplementary Fig. 2a-d, Supplementary Fig. 3a-b, Supplementary Table 3). These OSCs were therefore named lanostadienol synthases (LDSs). No activity was observed for the Parastichopus parvimensis sea cucumber candidate OSC PpLDS (Fig. 1e). GC-MS analysis of extracts from yeast expressing PpPS (from P. parvimensis), and AjPSa and AjPSb (from A. japonicus) revealed a new peak that did not match the retention time of either lanosterol (2) or lanostadienol (4) (Fig. 1f, Extended Data Fig. 2d, h), which was subsequently shown by NMR to be 8β-lanosta-9, 24-dienol (parkeol, 3) (Supplementary Fig. 3c, Supplementary Table 4). Parkeol (3) is another close isomer of lanosterol (2) and lanostadienol (4) with the relevant double bond at the Δ9(11) position. These OSCs were therefore named parkeol synthases (PSs). There is no report of a dedicated LDS as yet from any other organism. A single PS of unknown biological function has been reported from rice19. The identification of a dedicated PS in sea cucumbers is suggestive of a role for this enzyme in parkeol-type triterpene saponin biosynthesis as shown on the right side of Fig. 1b. The role of LDS in sea cucumbers is unknown.
Analysis of sea cucumber saponins
To investigate the roles of PS and LDS OSCs, we analysed different sea cucumber tissues for antifungal activity, saponins and OSC gene expression to establish whether expression of LDS/PS genes is correlated with bioactivity/saponin content. Sea cucumber saponins are highly antifungal2. We first evaluated extracts from different adult tissues of P. parvimensis and A. japonicus for inhibition of yeast growth, as a sensitive but indirect measure of saponin content. Strong antifungal activity was detected for extracts from the tentacles, body walls and tubefeet, but not for the intestines, muscles and male or female gonads, with similar results for both sea cucumber species (Fig. 2a, top panel).
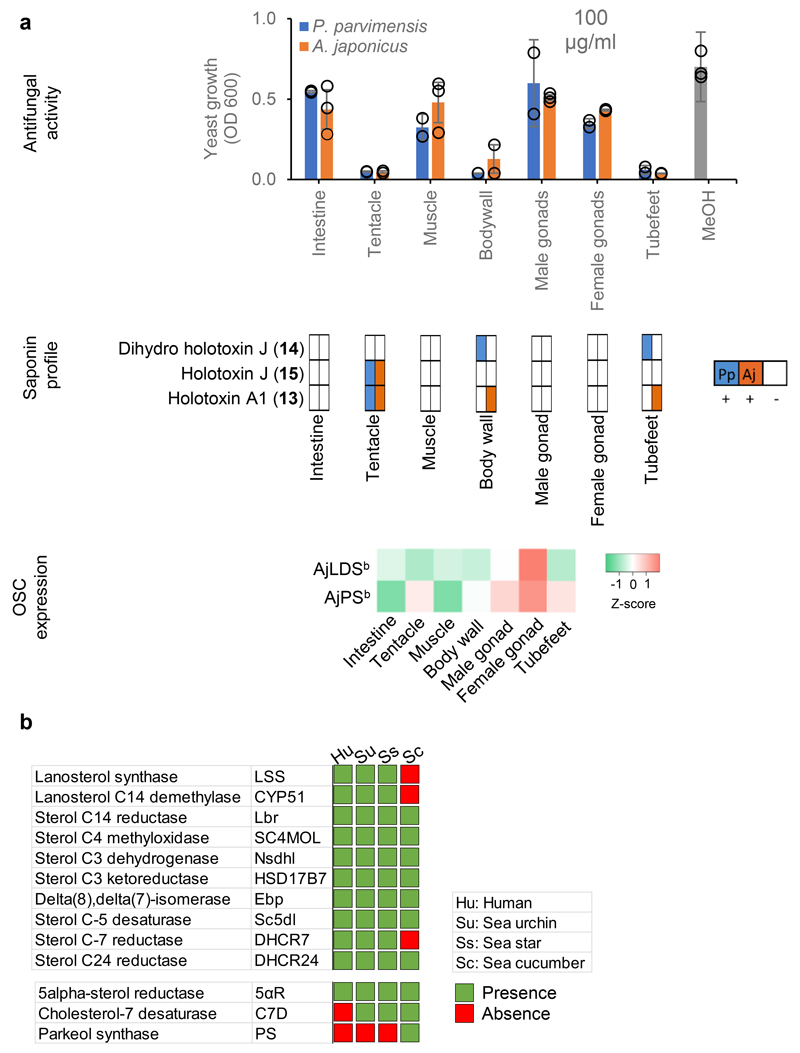
Fig. 2. Biosynthesis of defense saponins in sea cucumbers.
a, Antifungal activity, saponin profiles and OSC transcript levels for different sea cucumber tissues. Top, yeast growth (mean ± SD, Pp, n = 2; Aj, n = 3). Across all tissues 100 μg/ml crude extract was used with methanol (MeOH) as a control. Middle, presence (+) or absence (-) of saponins in P. parvimensis (Pp) and A. japonicus (Aj) based on LC-MS profiles (Extended Data Fig. 4a). Bottom, heatmap showing green (low) to red (high) OSC gene expression generated from RPKM values (Aj, n = 3). RPKM mean ± SD values are given in Source Data Fig. 2a. b, Comparison of sea cucumber (Sc) sterol biosynthetic genes with humans (Hu), sea urchins (Su), and sea stars (Ss). Presence or absence of sterol genes scored based on pairwise ortholog identity as shown in Supplementary Table 8.
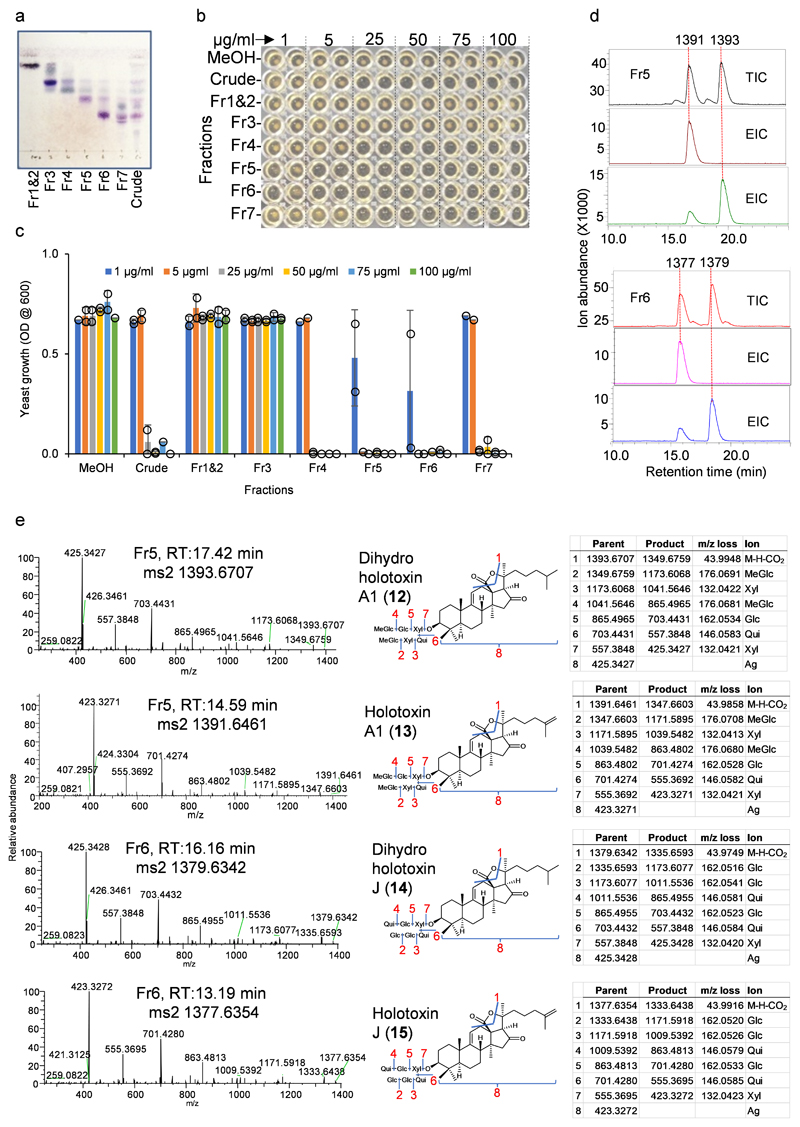
To facilitate saponin identification in crude extracts we isolated pure saponins from adult P. pavimensis sea cucumbers by large-scale extraction and purification, coupled with yeast growth inhibition and chromatographic analysis. Of the fractions tested, fractions 5 and 6 (fr5 and fr6) showed strong yeast growth inhibition (Extended Data Fig. 3a-c). These fractions each contained two major saponins, as revealed by LC-MS (-) (Extended Data Fig. 3d). These ions were further subjected to high-resolution LC-MS and MS2 analysis using aQ Exactive plus hybrid quadrupole-orbitrap mass spectrometer. Mass fragmentation of saponin m/z 1393.6707 revealed it to be dihydro holotoxin A1 (12) and m/z 1391.6461 as holotoxin A1 (13) (Extended Data Fig. 3e), previously known saponins from P. parvimensis20 and A. japonicus21. However, mass fragmentation revealed m/z 1379.6342 and m/z 1377.6354 to be new holotoxins (Extended Data Fig. 3e). These two new holotoxins each have two quinovose sugar residues as opposed to the single quinovose in all other currently known holotoxins20–21 and hence were named dihydro holotoxin J (m/z 1379.6342, 14) and holotoxin J (m/z 1377.6354, 15) (Extended Data Fig. 3e). All four saponins are parkeol-type with Δ9(11) functionality in the aglycone scaffold, and their structures are clearly consistent with high-resolution LC-MS2 fragmentation pattern shown in Extended Data Fig. 3e.
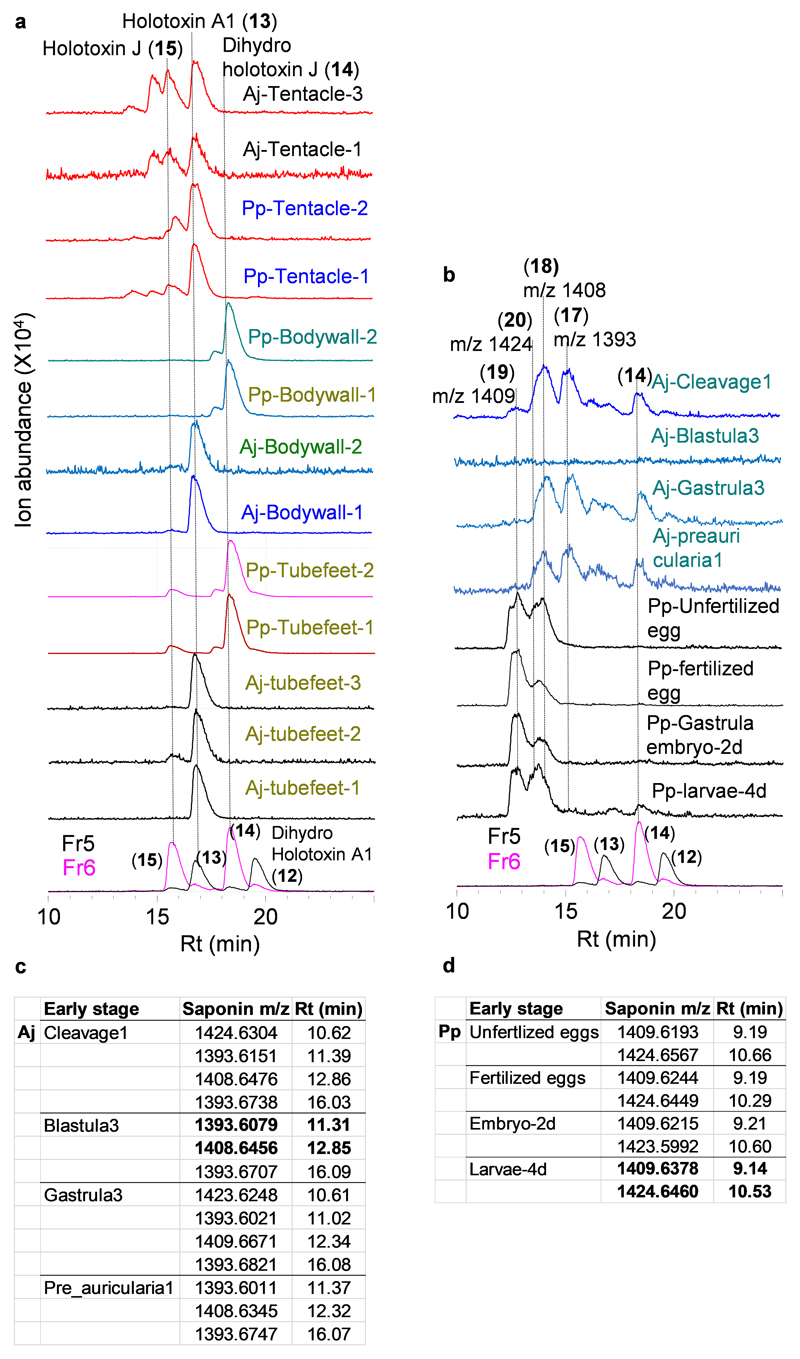
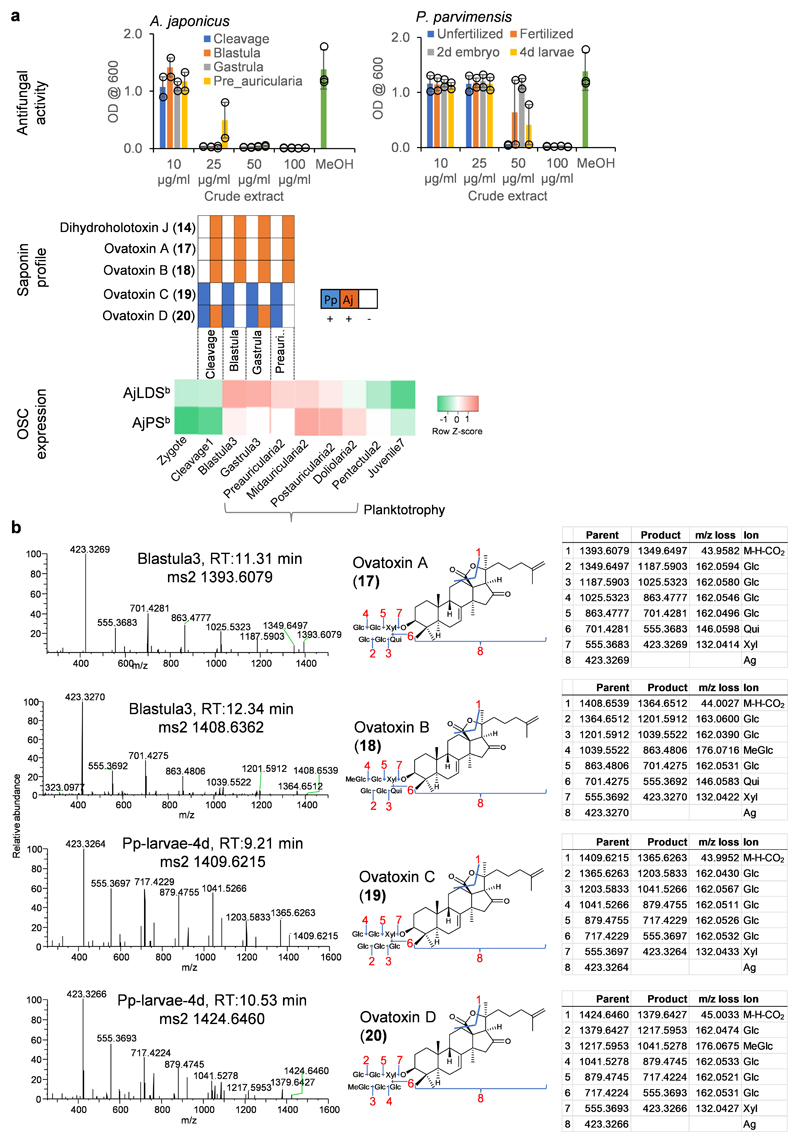
Using these saponins as standards we next analyzed the tissue extracts for saponin content. LC-MS2 analysis confirmed the presence of parkeol-type saponins with Δ9(11) functionality in the aglycone scaffold, consistent with a role for PS in saponin biosynthesis in these tissues (Fig. 2a; Extended Data Fig. 4a). These included dihydro holotoxin A1 (12), holotoxin A1 (13), dihydro holotoxin J (14) and holotoxin J (15). Holotoxin A1 (13) and holotoxin J (15) were found in extracts from the tentacles of both species. Holotoxin A1 (13) was also detected in extracts from the body wall and tube feet of A. japonicus, while dihydro holotoxin J (14) was predominant in extracts from the corresponding tissue types of P. parvimensis (Fig. 2a middle panel; Extended Data Fig. 4a). Selective accumulation of saponins in the outer epidermal tissues (i.e. tentacles, body wall, tube feet) may be anticipated to provide a first line of defense against pathogens or predators in the ocean. In fact, it has been shown that sea cucumber extracts inhibit the growth of surface-growing and pathogenic fungi of sea cucumbers, consistent with a direct role in defense22. Saponins were not detected in extracts from the intestines, muscles, and male or female gonads of either species, in line with the lack of antifungal activity in these extracts (Fig. 2a top and middle panel; Extended Data Fig. 4a). Analysis of the transcript levels of the two A. japonicus OSC genes revealed that AjPSb was expressed at high levels in the saponin-producing tissues while AjLDSb was not, consistent with a role for PS in saponin biosynthesis (Fig. 2a lower panel). Neither OSC gene was expressed in the intestines or muscles, tissues that lack detectable levels of saponins. In contrast, both AjPSb and AjLDSb were highly expressed in the gonads but gonad extracts had little or no inhibitory activity towards yeast, suggesting that PS and LDS may have alternate roles in this tissue (for example, in steroid hormone biosynthesis or other functions) (Fig. 2a).
The early growth stages of A. japonicus show distinct waves of expression of AjLDSb and AjPSb in the transition from zygote to juvenile (Extended Data Fig. 5a, lower panel). Expression of OSC genes during the planktotrophic stage of larval development, where larvae actively feed on algae and plankton in the top water column, could be associated with the production of saponins that counter predation. Compared to the adult stages, early growth stage extracts showed strong yeast growth inhibition () as well as distinct saponin peaks in LC-MS (Extended Data Fig. 4b-d, Extended Data Fig. 5, Supplementary Fig. 4). NMR structural characterization of these saponins was not feasible due to low abundance of the compounds and source material. However, based on high-resolution LC-MS and MS2 analysis and correlation with AjLDSb expression at these growth stages, compared to adult stages we identified these as new saponins, ovatoxins A-D (Extended Data Fig. 5b). Since ovatoxin A-D accumulation correlates with AjLDSb expression these compounds are likely LDS-type, with a Δ7(8) functionality in the saponin aglycone, providing evidence for a role for LDS in the biosynthesis of previously undescribed saponins in the early growth stages (Fig. 3). Under laboratory conditions sea cucumber eggs are known to be highly unpalatable to predatory fish and tunicates23, again suggesting a direct defense role of inherent saponins in the early growth stages. Collectively, these results indicate that PS and LDS have distinct roles in the biosynthesis of parkeol-type saponins with Δ9(11) functionality and LDS-type saponins with Δ7(8) functionality, respectively (Fig. 3), as part of the sea cucumber chemical defense arsenal.
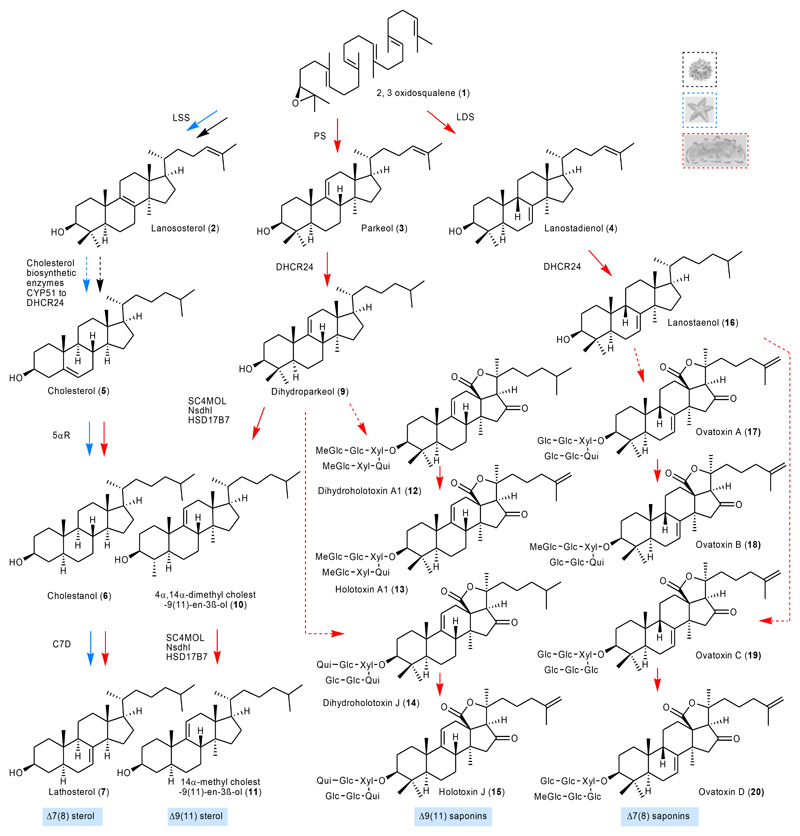
Fig. 3. Sea cucumbers make diverse triterpene saponins and unusual sterols.
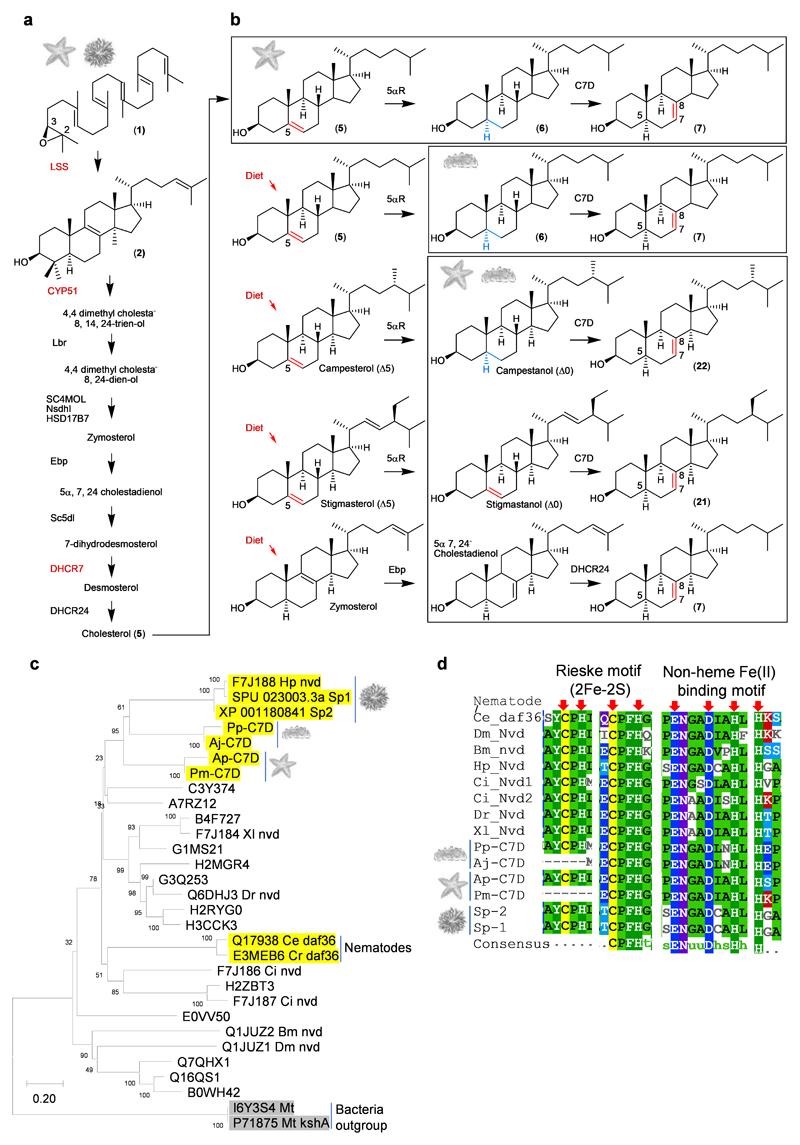
Roles of PS, LDS and C7D in the biosynthesis of unusual sterols [(Δ7(8)) and Δ9(11)] and of triterpene saponins [(Δ7(8) and Δ9(11)]. The different coloured arrows represent taxa-specific sterol or saponin biosynthetic routes. Solid and dotted arrows represent single and multi-step reactions, respectively. Pp, P. parvimensis; Aj, A. japonicus.
Sea cucumbers make unusual sterols
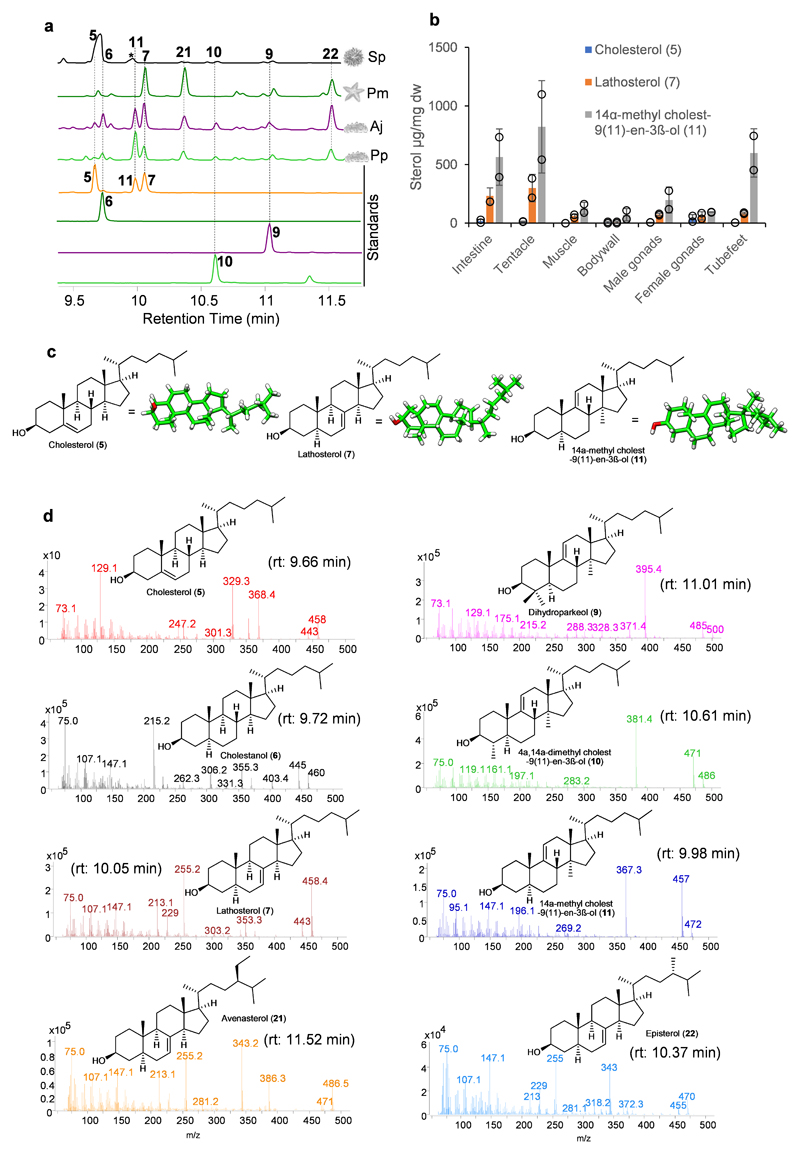
Saponins form complexes with cholesterol (5), so causing loss of membrane integrity and cell death. However, sea cucumbers have evolved the ability to produce high levels of lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterols rather than cholesterol (5), which may enable them to be resistant to their own saponins (Extended Data Fig. 6a, b). Sea urchins do not make saponins and lack lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterols (Extended Data Fig. 6a). External application of sea cucumber saponins to sea urchin eggs results in egg mortality24, 25. In contrast, application of sea star saponins to sea star eggs does not cause mortality, implying that intrinsic lathosterol (7) can confer resistance to saponin self-toxicity25. The alternative double bond positions {Δ7(8) and Δ9(11)} as well as conformation of acyclic tail of lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterols may hinder the formation of stable interactions with saponins in membranes when compared with Δ5 cholesterol (5) (Extended Data Fig. 6c).
The genes required for lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterol biosynthesis are currently unknown. Since knowledge of lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterol biosynthesis will be critical for understanding how sea cucumbers have evolved saponin resistance, we predicted the biosynthetic route to these sterols based on pathway intermediates characterized in this study and enzymes reported in the literature with the aim of identifying the corresponding genes (Extended Data Fig. 6a, d, Supplementary Fig. 5a-e, Supplementary Tables 5-8). Firstly, in 14α-methyl cholest-9(11)-en-3β-ol (11) biosynthesis, detection of dihydroparkeol (9) and 4α, 14α-dimethyl cholest-9(11)-en-3β-ol (10) (Extended Data Fig. 6a) intermediates implies that 14α-methyl cholest-9(11)-en-3β-ol (11) is derived from the triterpene precursor parkeol (3) through: a) side chain double bond reduction of parkeol (3) by a sterol C24 reductase (DHCR24) leading to dihydroparkeol (9); and b) eventual C4 demethylation of dihydroparkeol (9) mediated by the C4 demethylation complex (SC4MOL, Nsdh and HSD17B7), which results in formation of the final 14α-methyl cholest-9(11)-en-3β-ol (11) sterol (Fig. 3). 14α-methyl cholest-9(11)-en-3β-ol (11) sterol is unique to sea cucumbers, implying that it has a special role therein26. Secondly, in lathosterol (7) biosynthesis, in the absence of a cholesterol (5) biosynthetic pathway sea cucumbers must take up cholesterol (5) from their diet, whereas sea stars synthesize cholesterol (5) and also convert de novo synthesized or diet-derived Δ5 sterols to Δ7 sterols (Fig. 2b, Fig. 3; Extended Data Fig. 7a, b). Detection of cholestanol (6) suggests that cholesterol (5) 5α-reduction (mediated by a common 5α-sterol reductase: 5αR) precedes C-7 desaturation in lathosterol (7) biosynthesis (Fig. 3, Extended Data Fig. 6a). A literature search revealed that a bona fide cholesterol-7 desaturase (C7D, known as DAF36 in nematodes) is known in nematodes27. Using this as an enquiry sequence, we identified C7D hits in echinoderms but not in humans (Fig. 2b). Phylogenetic analysis reveals that the echinoderm hits group with the nematode DAF36 (Extended Data Fig. 7c). Key amino acid residues known to be required for function are also conserved, suggesting that the echinoderm hits are likely sterol C-7 desaturases (Extended Data Fig. 7d). C7D and PS play crucial roles in lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterols biosynthesis, respectively (Fig. 2b, Fig. 3). Collectively, our results suggest that PS and LDS have distinct and non-overlapping roles in vivo. To investigate this further, we carried out whole mount mRNA in situ hybridization of P. parvimensis embryos and larvae to establish the spatial expression patterns of the PS and LDS OSCs. In 2-day old embryos, PpLDS expression was detected in the ciliary bands, while PpPS was expressed in the mouth region, consistent with distinct roles for these two OSCs (Supplementary Fig. 6a-c).
Mutational analysis of determinants of OSC product specificity
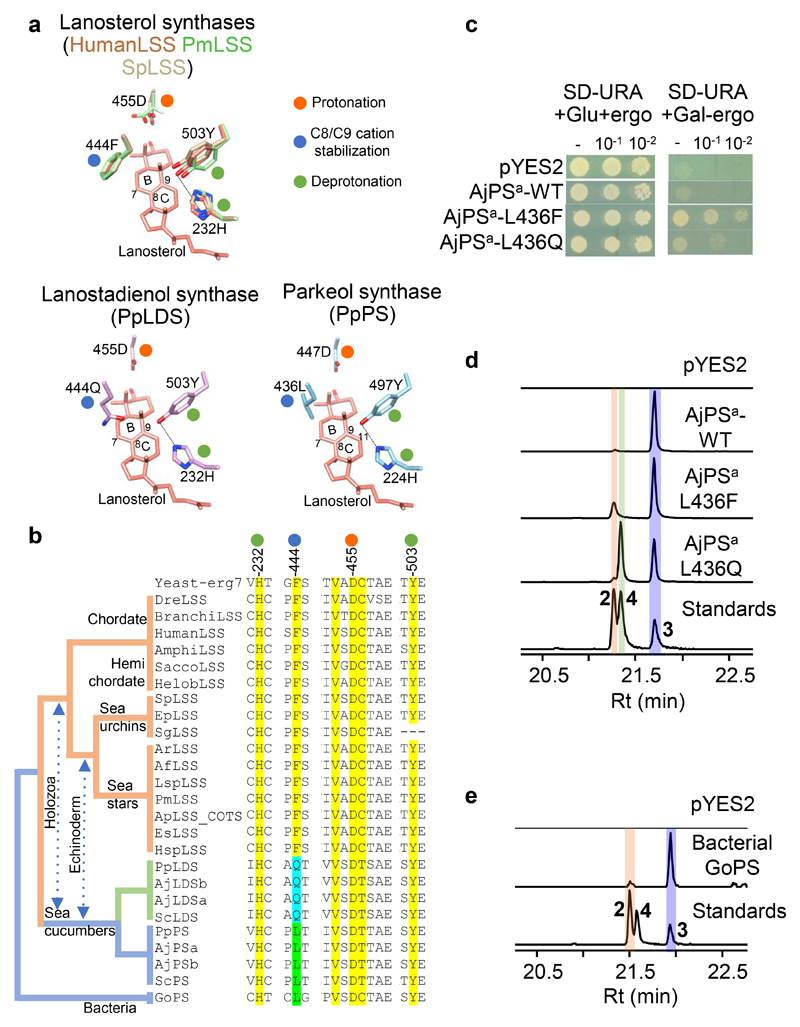
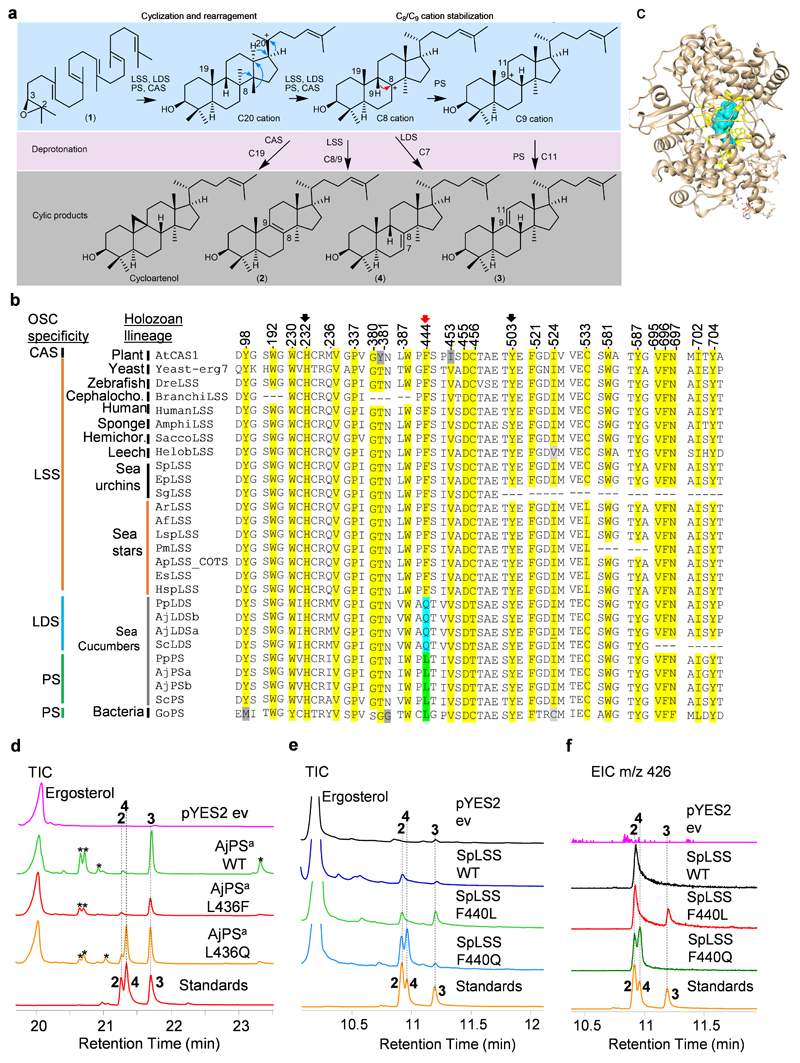
Our results suggest that PS is responsible for the first committed step in biosynthesis of Δ9(11) saponins and Δ9(11) sterols, and LDS for Δ7(8) saponin biosynthesis (Fig. 3). Unraveling the evolutionary origins of LDS and PS may shed light on the origin of Δ9(11) and Δ7(8) triterpene saponins and Δ9(11) sterols in sea cucumbers. To this end, functional divergence of the LDS and PS OSCs from the highly conserved ancestral LSSs prompted us to investigate the active site residues of these enzymes. The cyclization products generated by LSS, LDS and PS enzymes differ in the position of the double bond in the tetracyclic region. The different isomeric products are a result of different termination points of 1,2-shift sequences originating from a common tetracyclic cationic intermediate (the protosteryl cation). This implies that these OSCs likely differ/diverge either in their kinetic promotion of the final deprotonation (which gives the neutral alkene) or in their ability to stabilize the relevant preceding cations (Extended Data Fig. 8a). To determine whether these OSCs differ in active site amino acid residues that might govern the site of deprotonation or C8/C9 cation stabilization, we developed homology models for PmLSS, SpLSS, PpLDS and PpPS using the human LSS-lanosterol (pdb; 1w6K) complex as a template28. Superimposition of the models reveals a single amino acid residue within 5Å of lanosterol that differs in LSS (444F), LDS (444Q) and PS (436L) (Fig. 4a, Extended Data Fig. 8b). This revealed that residue 444 is in a particularly promising position to control C8/C9 cation stabilization directly, which in turn could influence the position of deprotonation during the reaction pathway (Fig. 4a). Next, using structure-based sequence alignment we verified variability at 444-residue position across the echinoderm as well as the Holozoan lineages. Comparison of amino acid residue positions within 5Å of lanosterol (2) (highlighted in yellow) revealed that residue 444 alone differentiated LDSs and PSs from LSSs (Fig. 4b, Extended Data Fig. 8b-c). Residues 232H and 503Y are within hydrogen bonding distance and are believed to form a catalytic dyad responsible for deprotonation in the formation of lanosterol (2)28 (Fig. 4a). H232 and 503Y are invariant across LSS LDS PS OSCs, suggesting that variation at residue 444 alone determines product specificity (Fig. 4b). Natural selection may therefore have favored this mutation, leading to the origin of the divergent OSCs, LDS and PS, and to the emergence of the Δ9(11) (12-15) and Δ7(8) (17-20) triterpene saponins as well as 14α-methyl cholest-9(11)-en-3β-ol (11) sterol in sea cucumbers.
Fig. 4. A single active residue underlies functional divergence of LDS and PS from LSS in echinoderms.
a, Super-positioned homology models of sea star (PmLSS), sea urchin (SpLSS) and divergent sea cucumber OSCs (PpLDS and PpPS) showing variation at position 444 position near B/C rings of lanosterol. Coloured circles next to amino acid residues in the models represent different cyclization roles. Dotted lines represent a hydrogen bond between Y503 and H232, and numbering on B/C rings of lanosterol represent cationic regions. b, Structure based sequence alignment of position 444 and its associated positions across echinoderm and Holozoan OSCs. Clades coloured according to OSC product specificity. OSC sequence numbering is according to Human LSS. Further information about sequences used is provided in Supplementary Table 2. c, Complementation of LSS-deficient yeast strain with AjPSa-WT and mutants thereof. From stock cultures yeast was spotted undiluted (-), and diluted 10 and 100-fold. d,e, GC-MS profiles of yeast extracts from strains expressing AjPSa-WT and active site mutants (d), and the bacterial OSC GoPS (e; Gemmata obscuriglobus parkeol synthase). The corresponding TICs for d and e are shown in Extended Data Fig. 8d and Extended Data Fig. 9c, respectively. Standards; lanosterol (2), lanostadienol (4) and parkeol (3)
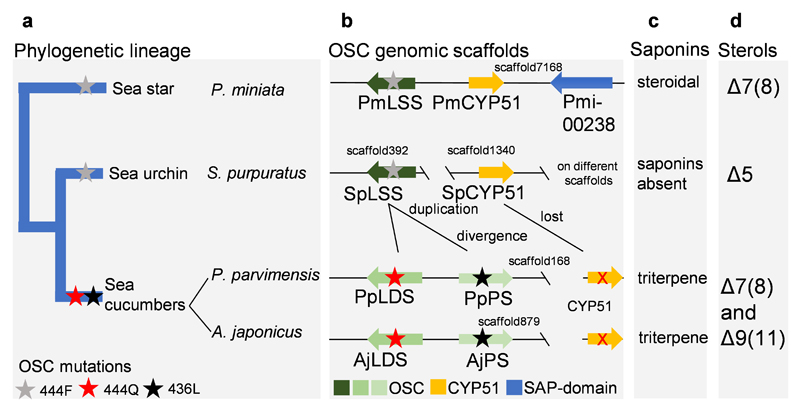
Next, we examined experimentally, in three ways, whether the nature of the amino acid residue at position 444 does indeed determine the product specificity of LSS/LDS/PS enzymes. Firstly, an amino acid residue in the structurally homologous position was swapped with a residue from the equivalent position of another OSC. AjPSa was chosen as a representative PS and mutant versions with the equivalent LSS or LDS residues (AjPSa-L436F and AjPSa-L436Q) were generated and expressed in yeast. As expected, the wild type AjPSa enzyme neither restores the ability of Gil77 to grow in the absence of exogenous ergosterol, nor yields lanosterol (2) (Fig. 4c-d, Extended Data Fig. 8d). However, the mutant AjPSa-L436F variant can restore the growth of Gil77 (Fig. 3c), indicating that it is able to produce lanosterol (2) in vivo. GC-MS results confirmed the presence of lanosterol (2) in extracts from yeast expressing this mutant PS (Fig. 3d, Extended Data Fig. 8dd). The mutant variant AjPSa-L436Q makes lanostadienol (4) in addition to parkeol (3), but not lanosterol (2) (Fig. 4d, Extended Data Fig. 8d). Secondly, we introduced corresponding mutations in SpLSS to see if these mutations recapitulate PS-or LDS-like activity in LSS. The SpLSS wild type enzyme does not make detectable levels of parkeol (3) or lanostadienol (4). Interestingly, however, the SpLSS-F440L mutant makes lanostadienol (4) in addition to lanosterol (2) and the SpLSS-F440Q mutant makes lanostadienol (4) in addition to lanosterol (2) (Extended Data Fig. 8e-f). Thirdly, we investigated whether any other OSCs in the UniProt proteome29 have Q or L at the position equivalent to 444 instead of the conserved F. An exhaustive search revealed five hits, all with L at this position (Extended Data Fig. 9aa). These five hits, all of which are of unknown function, fall into the bacterial group I OSCs based on our phylogenetic analysis (Extended Data Fig. 9b). We cloned one of these, from Gemmata obscuriglobus strain DSM5831T, (GoOSC) and expressed it in yeast. GC-MS analysis revealed that GoPS makes parkeol (3) as we predicted, confirming the pivotal role of 444L in parkeol (3) product specificity (Fig. 4e, Extended Data Fig. 9c). Together our results clearly implicate residue 444 in OSC product specificity and are consistent with a scenario in which LDS/PS enzymes have evolved from an ancestral sea cucumber LSS by gene duplication and neo-functionalization. The PS and LDS genes are in tandem in the P. parvimensis and A. japonicas sea cucumber genomes, suggesting that they may have arisen by gene duplication and divergence, and this may have occurred in parallel with CYP51 loss in sea cucumbers (Extended Data Fig. 10a-d). CYP51 (sterol 14α demethylase) enzymes are highly conserved across the animal, plant and fungal kingdoms, and govern a key step in essential sterol biosynthesis30. However, unlike sea stars and sea urchins, sea cucumbers lack a CYP51 gene.
Discussion
Here we show that sea cucumbers lack LSS, an OSC that is essential for normal sterol biosynthesis in animals and that is highly conserved across other members of the animal kingdom. Instead, they have two divergent OSCs (parkeol synthase, PS; and lanostadienol synthase, LDS) that respectively produce parkeol-type triterpene saponins in the epidermal tissues and a new class of lanostadienol-type saponins (ovatoxins) in the juvenile tissues, and that are likely to have evolved from an ancestral LSS by gene duplication and neo-functionalisation. Saponins are able to form complexes with membrane sterols, so causing membrane disruption with associated cell death. Our results show that sea cucumbers produce high levels of the unusual sterols lathosterol (7) and 14α-methyl cholest-9(11)-en-3β-ol (11) sterols, rather than the typical animal sterol, cholesterol (5). These sterols are saponin-resistant, and hence are likely to confer protection against self-poisoning. Collectively out studies suggest that sea cucumbers have evolved the ability to produce saponins as well as saponin-resistant sterols concomitantly. The discovery of the key enzymes LSS, PS, LDS and C7D should now expedite the discovery and characterization of other downstream enzymes required for saponin biosynthesis in sea cucumber.
Methods
Sea cucumber sampling and maintenance
Culturing and harvesting of adult tissues and early stages of Parastichopus parvimensis
Adult animals of sea cucumbers P. parvimensis were collected off the southern California coast, USA. Spawning was induced in adult sea cucumbers by intra-coelomic injection of 100 nM NGLWY-amide followed by heat shock in room temperature sea water as described previously31. Fertilization was conducted by mixing freshly shed eggs with dilute sperm. Embryos were cultured in artificial seawater at 15°C until the desired stage. Late gastrula/larva stage embryos were collected at 2 days post fertilization (dpf) and early auricularia larvae were collected at 4 dpf. Nine different adult tissues were collected from P. parvimensis sea cucumbers and flash-frozen until further use.
Culturing and harvesting of adult tissues and early stages of Apostichopus japonicus
Adult A. japonicus sea cucumbers were collected from the coast of Liaoning, China and acclimatized in a seawater aquarium (~500 l) at 15°C for one week before use. They were fed once a day during this period with mixed feed ingredients including 40% fresh sea mud, 30% Sargassum thunbergii and 30% sea cucumber compound feed (An-yuan company). Major adult organs (intestine, tentacle, muscle, body wall, male gonad, female gonad and tube feet) were dissected from three healthy adult sea cucumbers and flash-frozen in liquid nitrogen for further use. To obtain the early-stage materials of A. japonicus, artificial fertilization of sexually matured adults was performed as described previously32. Larval culture was developed based on procedure described previously32. Embryos (zygote, cleavages, blastulae and gastrulae), larvae (auricularia, doliolaria, and pentactula) and juveniles were sampled and flash-frozen until use.
Oxidosqualene cyclase (OSC) mining
Genome and transcriptome resources14–17, 33, 34 were used to mine for predicted echinoderm OSC sequences. EchinoBase16 hosts genome sequences and ovary transcriptome data for diverse species of Echinodermata, including sea urchins, sand dollars, sea stars, and sea cucumbers. These available echinoderm sequence resources16 were mined using the human lanosterol synthase sequence as a template (Uniprot id: P48449)28 and predicted full-length OSC open reading frames (ORF) (~2.2 Kb; ~720 amino acids) were recovered. In cases where only partial OSC hits were identified, multiple small contigs were assembled to give full-length OSC ORFs. Contig assembly was performed using the ContigExpress tool in Vector NTI (v11.5.3) and ORF or gene prediction was carried out using FGENESH35. In this study two different accessions of A. japonicus were used for OSC mining. Sequence resources for the accession ‘a’ were derived from EchinoBase16 and Reich et al33 (BioProject accession no. PRJNA236087). We recovered two OSCs from this accession and were annotated with superscript ‘a’ throughout the study. The A. japonicus accession ‘b’ was derived from Li. Et al. (2018)14 (BioProject accession no. PRJNA413998). OSC hits from this accession were annotated with superscript ‘b’. A predicted full-length lanosterol synthase from EchinoBase (SpLSS)16 was also assembled from sea urchins. All contigs used in full-length OSCs assembly and their associated details are listed in Supplementary Tables 1 and 2. Further, predicted OSC amino acid sequences were scanned for the presence of the key active site motif DCTAE, which is implicated in protonation of 2,3-oxidosqualene and initiation of cyclization reaction36.
Phylogenetic analysis
OSC amino acid sequences were aligned using ClustalW (ver2.1) with default parameters as implemented in the program MEGA7 (ver10.2.6)37 and the CLC Genomics Workbench (v9.5.3). Positions with gaps and missing data were eliminated. Evolutionary distances were computed using the JTT matrix-based method38. Phylogenetic analysis of OSC sequences was carried out using a neighbor-joining tree with 1,000 bootstrap replicates39.
Cloning
PmLSS of Patiria miniata was cloned from embryo (2d old) cDNA. PpOSC1 and PpOSC2 were cloned from mixed cDNA of P. parvimensis early developmental stages (embryo 2d, larvae 4d). These were PCR-amplified using primers with attB1 and attB2 adapters and cloned into pDONOR207 (GenR, Invitrogen) through a BP recombination reaction. PCR conditions were as follows: initial denaturation (95°C, 1 min); 30 cycles of 95°C for 30 s; 58°C for 1 min; 72°C for 4 min; final elongation step of 7 min at 72°C. The PCR reaction mix contained: 2μl cDNA; 10 μM of each primer; 250 μM of each dNTP; 1x Phusion buffer; and 1U of Phusion DNA polymerase (NEB). After BP reaction and transformation colony PCR was performed using GoTaq® Green Master (Promega) and positive clones were validated by sequencing. Positive clones were sequenced using three sets of primers covering entire length of the gene (~2.2 Kb). The bacterial OSC candidate was cloned from genomic DNA of Gemmata obscuriglobus DSM5831T strain (GoOSC). Error free clones were then cloned into pYES2 (AmpR) through yeast homologous recombination. All primers used are listed in Supplementary Table 9 (from Sigma-Aldrich).
Gene assembly using yeast homologous recombination
The SpLSS, AjOSC1a, AjOSC2a, AjOSC1b and AjOSC2b coding sequences were synthesized as gBlocks® by Integrated DNA Technologies (IDT). The gBlocks were then recombined to give full-length genes by homologous recombination in yeast40. All yeast homologous recombination and expression analysis was carried out in the yeast strain Gil77 (gal2 hem3-6 erg7 ura3-167)18. The gBlocks® were PCR amplified using the primers listed in supplementary table 9. Each primer contained a region that overlaps with the pYES2 vector sequences with 5’ end of the forward primer overlaps with the GAL1 promoter, and the 5’ of the reverse primer overlaps with the CYC1 terminator. Whereas 3’ ends of the primers overlap with the beginning and end of the respective gBlocks. Amplified gBlocks were then co-transformed into Gil77 together with XbaI- and HindIII- (Invitrogen) linearized pYES2 vector (AmpR, Invitrogen). Yeast transformation was performed using a standard protocol (Yeastmaker™ Yeast transformation system 2). Transformation resulted in in vivo recombination between the pYES2 vector and the gBlock OSC fragments leading to generation of full-length expression constructs. Plasmids were recovered from the yeast, transformed back into E. coli (DH5α) and their sequences were verified through sequencing. All primers used are listed in Supplementary Table 9.
Yeast expression
Yeast expression was carried out using the strain Gil7718. Yeast strains containing different expression constructs were grown in selective medium (SD-URA+ 2% glucose + supplements) (5ml) at 28° C until saturation (~2 days). The supplements included: ergosterol (Fluka), 20 µg/ml; hemin (Sigma-Aldrich), 13 µg/ml; and Tween-80 (Sigma-Aldrich), 5mg/ml. Next cells were pelleted, washed in dH2O (5 ml), transferred to induction medium (SD-URA+2% galactose) and incubated for a further 2 days to allow OSC expression and accumulation of triterpenes. Yeast pellets were washed once with dH2O and stored at -80°C until extraction.
Yeast lanosterol synthase complementation
Gil77 is a lanosterol synthase (LSS) mutant and is unable to grow in the absence of exogenously supplied sterol (ergosterol)18. This phenotype can be rescued by complementation with a lanosterol synthase OSC, since OSC-derived lanosterol is converted to ergosterol by endogenous yeast enzymes. For complementation analysis, Gil77 OSC transformants were spotted onto the following media in 10-fold serial dilutions; a) SD-URA + glucose plates supplemented with ergosterol, Tween-80 and hemin; and b) SD-URA + galactose without ergosterol. The GAL1 promoter of the pYES2 construct drives gene expression in the presence of galactose and is repressed in the presence of glucose. Thus, any OSC that makes lanosterol in vivo is expected to complement Gil77 growth in the absence of exogenous ergosterol and presence of galactose.
Triterpene extraction
Frozen yeast pellets (~50mg, fw) were mixed with 0.5 ml saponification reagent (20% KOH in 50% ethanol, v/v) and incubated at 65°C for 2 h before extraction with an equal volume of hexane (0.5 ml). The hexane extraction was repeated (2x) to maximize triterpene recovery. The combined extract was dried under nitrogen gas and the residue dissolved in 0.5 ml n-hexane. For rapid qualitative analysis, extracts were run on thin layer chromatography (TLC) plates (Catalog# 70644, FLUKA) using a hexane: ethyl acetate (6:1, v/v) solvent system. Compounds were visualized by spraying the plates with acetic acid: H2SO4: p-anisaldehyde (48:1:1, v/v/v) and heating to 120°C for 5 min on a TLC plate heater (CAMAG).
GC-MS analysis
For GC-MS analysis, 100 µl aliquots of n-hexane extracts from yeast experiments were transferred to 150 µl vial inserts and analysed using an Agilent GC (7890B)-MSD (5977A) with a robotic multi-purpose auto-sampler (MPS). Samples were run on an HP-5MS column (30 m x 0.25 mm, id; 0.25 µm film, Phenomenex). The injector port, source and transfer line temperatures were set at 250°C. An oven temperature program from 80°C (2 min) to 290°C (30 min) at 20°C/min was used. The carrier gas was helium; the flow rate was 1.2 ml/min. Samples (2 µl) were injected in split less mode. For sterol quantification the following gradient was used. Pulse pressure was set at 20 psi for 0.5 min after injection and the inlet was purged after 0.5 min with a split vent flow at 100 ml/min. Samples (2 µl) were injected in a pulsed split mode (5:1 split ratio). The injector port, source and transfer line temperatures were set at 250°C. An oven temperature program from 150°C (2 min) to 270°C (6.5 min) at 20°C/min was used, followed by 300°C at 4°C/min over 14 min, ramping up to 360°C at 40°C/min and then holding at 360°C for 7 min. The carrier gas was helium and the flow rate was 1.0 ml/min. The output was used to search the NIST (v8) library to assign identity to common peaks in the GC-MS traces. All experiments were repeated to confirm reproducibility of the triterpene profiles.
Inhibition of endogenous yeast erg11
In LDS OSC experiments ketoconazole was used to control undesired OSC product modifications by the endogenous yeast enzyme ERG11. Ketoconazole is a lanosterol-14α demethylase (ERG11/CYP51) inhibitor. Ketoconazole (Catalog# K1003, Sigma-Aldrich) was dissolved in DMSO and used in SD-URA+2% galactose media (50 µg/ml = ~100 µM).
Triterpene standards
Lanosterol (2) was purchased commercially (Catalog# L5768, Sigma-Aldrich), while lanostadienol (4) and parkeol (3) were purified in the present study (see below). These compounds were dissolved and diluted to 0.5 mg/ml in n-hexane and used as standards in GC-MS analysis.
Purification of (3) and (4) from yeast
Yeast strains expressing LDS or PS were grown (5L) in minimal media (SD-URA+ 2% glucose + supplements) and induced (SD-URA+2% galactose) for two days as described above. Cells were then pelleted by centrifuging at 4000 rpm for 15 min (SLC6000 rotor, Sorval Evolution) and washed once with dH2O (500 ml) prior to extraction. Washed cell pellets (~50-60 g fresh weight) were resuspended in 500 ml of saponification reagent (20% KOH in 50% ethanol, v/v) and incubated in a water bath (65°C for 2 h) before extraction with an equal volume of hexane. Hexane extraction was repeated (3x) to maximize triterpene recovery. The extract was then dried in vacuo using a rotary evaporator. The organic residue (~ 200-300 mg) was dry loaded onto pre-made silica columns (Biotage® SNAP Ultra 10g, 21 x 55 mm, 25µm) that had been equilibrated with hexane. Separation was carried out using an advanced automated flash chromatographic purification system Isolera™ One (Biotage). Chromatographic runs were carried using a gradient of ethyl acetate (solvent B) in hexane (solvent A). Gradient elution was initiated with hexane for 10 column volumes, switched to ethyl acetate in hexane (2% to 30%, v/v) over 25 column volumes and fractions (5ml) were collected throughout the run. Fractions were assessed for triterpene content using TLC (silica gel 60 F254 plates, Merck). Those containing the relevant triterpenes were pooled and purity assessed by GC-MS (as described above). Based on the purity, purification was repeated to ensure clear separation of closely resolving compounds. Fractions containing pure (3) and (2) were combined, dried using a rotary evaporator (Buchi), and subjected to NMR.
NMR analysis
1D and 2D NMR spectra (1H, 13C, DEPT135, COSY, HSQC, HMBC and NOESY) were acquired on a Bruker 400 MHz Topspin NMR spectrometer. All signals were acquired at 298 K. Samples were dissolved in deuterated chloroform (CDCl3) for data acquisition and calibrated by referencing to either residual solvent 1H and 13C signal or TMS. Compound identities were confirmed by comparing their 1H and/or 13C NMR data with those reported previously in the literature41–42.
Large-scale saponin extraction and purification from processed sea cucumbers
Processed P. parvimensis sea cucumbers (~1.75 kg dry weight) (WK Distribution CA, USA) were soaked in distilled water for 2 d to allow swelling and softening. Soft and swollen sea cucumbers were pulverized and soaked in 100 % ethanol (v/v) for two weeks. Ethanol extract was filtered (Whatman No. 1 filter paper) and dried in vacuo using a Rotavapor R-300 (Buchi) at 30°C. The remaining aqueous layer was extracted with n-hexane. The n-hexane extract was used in purification of sterols as described below. After n-hexane extraction the aqueous extract was partitioned with n-butanol (1:5 ratio, v/v) and centrifuged (4000 rpm, SLC6000 rotor, Sorval Evolution) to enable phase separation. N-Butanol partitioning was repeated (3x) to ensure efficient recovery of saponins. The combined n-butanol extracts were dried under vacuum at 56°C using a Rotavapor R-300 (Buchi). The resulting extract (greasy yellowish) was freeze-dried to remove any residual n-butanol. Freeze-dried extract (~3 g) was dry loaded onto a normal phase silica column (Biotage® SNAP Ultra 100g, 39 x 157 mm, 25 µm) and separated using an advanced automated flash chromatographic purification system (Isolera™ One). Separation was carried out with a gradient of dichloromethane (DCM, solvent A) and methanol (7.5% H2O in methanol, solvent B). Run was initiated with DCM, switched to 15% methanol (v/v) in DCM (v/v) over 50 column volumes, and then switched to 30% methanol (v/v) in DCM (v/v). During this process 22 ml fractions were collected, dried and analysed for saponins by TLC (Silica gel 60G glass plates 20 x 20 cm, Merck) using avenacin A-1 from oats43 as a tracking standard. Saponins were visualized by spraying the plates with acetic acid: H2SO4: p-anisaldehyde (48:1:1, v/v/v) and heating to 120°C for 5 min on a TLC plate heater (CAMAG).
Yeast growth inhibition assays
Yeast growth inhibition assays were carried out using yeast strain Y21900 (BY4743; MATa/MATα; ura3Δ0/ura3Δ0; leu2Δ0/leu2Δ0; his3Δ1/his3Δ1; met15Δ0/MET15; LYS2/lys2Δ0; YHR072w/YHR072w::kanMX4, Euroscarf, Germany). Y21900 was grown in YPD media. A stock solution of crude extracts and saponin standards (1 mg/ml) were prepared in methanol and by serial dilution lower concentrations were prepared. All yeast growth inhibition assays were carried out in 96-well plates (skirted, round bottom, Sterilin 96). Adult tissues and early growth stages of P. parvimensis (n = 2) and A. japonicus (n = 3) were used in the analysis. Two technical replicates were also included to account for low volume pipetting errors etc. Sea cucumber early developmental stages consisted of several hundred embryos or larvae per sample. Assays were carried out in a 100 µl YPD media seeded with 1 µl yeast culture (100-fold dilution of an overnight culture). Saponins (1 to 100 µg/ml, 1-5 µl) and crude extracts (25 to 100 µg/ml, 1-5 µl) were added at the beginning of the culture period and plates were incubated in a shaking incubator (30° C overnight, 100 rpm). Growth was recorded by measuring optical density (OD600) using a FLUOstar Omega multidetector microplate reader (BMG labtech) with default settings. Sample OD values were corrected against OD values of wells containing YPD medium alone (called blank corrected).
Saponin analysis and characterization using Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer
Ultra-high-performance liquid chromatography/mass spectrometry (UHPLC/MS) analysis of the saponins was carried out on Q Exactive (Thermo Scientific). Chromatography was performed using a Kinetex 2.6 μm XB-C18 100 Å, 50 mm x 2.1 mm (Phenomenex) column kept at 30°C. Water containing 0.1% formic acid (FA) and acetonitrile containing 0.1% formic acid (FA) were used as mobile phases A and B, respectively, with a flow rate of 0.3 mL/min and 10 µL injection volume. A gradient elution program was applied as follows: 0-5 minutes linear increase of 0 to 30% B, 5-28 min linear increase of 30 to 50% B, 28-33 min linear increase of 50 to 100% B, and from 33-34 min linear drop from 100 to 20% B 1 min hold on for re-equilibration, giving a total run time 35 min. Full scan in combination with data-dependent MS2 scans (Full MS/dd-MS2, Top 3) was applied. MS detection was performed in negative ionization ESI range of 100 to 2000 m/z and mass resolution of 70,000. Targeted selected ion monitoring (tSIM) and parallel reaction monitoring (PRM) acquisition were performed with mass resolution set at 35,000 FWHM and a mass isolation window of 1.6 m/z and AGC of 3e6. In PRM mode, the data were acquired according to a predetermined inclusion list containing the accurate masses of known saponins. Data was acquired and processed utilizing Thermo Scientific™ Xcalibur software (ver4.3.73.11). Saponin mass fragmentation spectra were compared with previously reported saponins from P. parvimensis20 and A. japonicus21.
Small-scale saponin extraction from adult tissues and early stages
To establish if saponin accumulation is tissue-specific, in a small scale, saponin activity and saponin content were analyzed by yeast growth inhibition assays and LC-MS over a range of sea cucumber tissues and early growth stages. Seven adult tissues were collected from two different species of sea cucumbers, P. parvimensis (n = 2) and A. japonicus (n = 3). Tissue samples were harvested into 1.5 ml Eppendorf tubes, freeze-dried, and homogenized using GenoGrinder (1500 rpm, 1 min). A known weight of homogenized tissues (~2-3 mg d.w.) were extracted twice with methanol (200 μl) in a sonication bath (EMAG ultrasonic cleaner Emmi 12 HC, 45 min). Combined extracts were centrifuged (14,680 g, 5 min) and supernatants transferred to pre-weighed 1.5 ml Eppendorf tubes. Extracts were dried in vacuo using a Rotavac (EZ-2 Series Evaporator, Genevac) at 30°C for 45 min. Samples were then freeze-dried to remove any residual methanol. Stock solutions (1 mg/ml and 100 μg/ml, wt/v) were prepared and serially diluted in methanol.
LC-MS analysis of extracts from adult tissues and early stages
An LC method was designed with a flow rate of 0.3 ml/min (2.6 mm XB-C18 100 Å, 5032.1 mm) (Phenomenex). The solvents used were solvent A, H2O + 0.1% formic acid: and solvent B, acetonitrile + 0.1% formic acid. Sea cucumber adult tissues and early-stage extracts (100 μg/ml) were analyzed using LC-MS-CAD (Instrument: Prominence HPLC system, single quadrupole mass spectrometer LC-MS-2020, Shimadzu) both in positive and negative ion modes. The gradient was as follows: 30% B, 0-5 min; 20- 50% B from 5- 28 min; 50- 100% B from 28- 30 min; 100% B from 30- 33 min; 100- 20% B from 33- 34 min; 20% B from 34- 35 min. Injection volume: 10 µL. LC-MS LabSolutions (ver3) (Shimadzu) was used to analyze chromatograms.
Purification and characterization of unusual sterols from sea cucumbers
Crude n-hexane extract recovered from phase separation during large-scale saponin extraction was dried in vacuo in a rotovapor (Buchi). Around 2 g of the greasy yellowish crude extract was dry-loaded onto a normal phase silica column (Biotage® SNAP Ultra 100g, 39 x 157mm, 25µm) that had been pre-equilibrated with n-hexane. Separation was carried out using Isolera™One (Biotage). Chromatographic separation was carried with a gradient of 0-3% ethyl acetate (solvent B) in n-hexane (solvent A) over 60 column volumes, followed by a gradient of 3 -20% solvent B over 10 column volumes. Fractions (22 ml) were collected and evaluated by TLC as described earlier. Fractions with sterols of interest were pooled and purity was assessed by GC-MS analysis as described earlier. Purified compounds were subjected to NMR in deuterated chloroform (CDCl3, Sigma-Aldrich).Compound identities were confirmed by comparing their 1H or 13C NMR data with those reported previously44, 45–50.
Analysis of sterols from sea urchins, sea stars and sea cucumbers
Sterols were extracted from different adult tissues of sea cucumbers, sea urchins and sea stars (2 mg, dw). Dried tissues were extracted in ethyl acetate (100 µl) with 10 µg/ml internal standard (hexatriacontane-d74, Sigma-Aldrich) in a sonicated water bath (EMAG ultrasonic cleaner Emmi 12 HC, 30 min). Extracts were centrifuged (2000 rpm, 2 min) and the supernatants dried under nitrogen gas for 15 min. The dried residues were derivatized using 1-(trimethylsilyl) imidazole-pyridine mixture (TMS) (Catalog# 92718, Sigma-Aldrich) at 75°C for 30 min. Next samples were transferred to 150 µl vial inserts and analyzed in GC-MS using a 14-min gradient as described earlier in earlier sections. Standards cholesterol (5) (Sigma-Aldrich), lathosterol (7) (Sigma-Aldrich) and 4α, 14α-dimethyl cholest-9(11)-en-3β-ol (11) (this study) were also derivatized in similar manner with 10 µg/ml of internal standard and used in the study.
Sterol quantification
A series of different concentrations of cholesterol (5), lathosterol (7) and 4α, 14α-dimethyl cholest-9(11)-en-3β-ol (11) were prepared in n-hexane from a 1 mg/ml stock. Ion m/z 66 was used as a quantifier for the internal standard. Ions m/z 129, 459 and 457 used as quantifiers for cholesterol (5), lathosterol (7) and 4α, 14α-dimethyl cholest-9(11)-en-3β-ol (11) sterols, respectively. Calibration curves of all sterol standards had a regression coefficient of R2>0.99. Qualifiers were automatically selected by the MS quantitative analysis software (Agilent). Automated data generated by MS quantitative analysis software was confirmed by manual inspection of peaks mass spectra and retention times. Calibration curves and quantification was performed using MS Quantitative Analysis software as implemented in MassHunter Chemstation (verB.07.00) (Agilent).
AjLDS and AjPS RNA-seq expression analysis
A.japonicus adult organs (intestine, tentacle, muscle, body wall, male gonad, female gonad and tubefeet), embryonic stages (zygote, cleavages, blastulae and gastrulae), larvae (auricularia, doliolaria, and pentactula) and juvenile samples were used for RNA-seq libraries construction. Total mRNA for each tissue type was extracted following the protocol described earlier51. RNA-seq libraries were constructed using the NEB Next mRNA Library Prep Kit (NEB, USA), following the manufacturer’s instructions. The prepared libraries were subjected to paired-end 100-bp (PE100) sequencing using the Illumina HiSeq 2000 platform. Sequencing reads were first filtered by removing those containing undetermined bases (‘N’) or excessive numbers of low-quality positions, and then aligned to the A. japonicus genome using STAR aligner with its default parameters52. Raw counts of aligned reads per gene were obtained using HTSeq (ver0.6.1)53. Gene expression levels were represented in the form of reads per kilobase of exon model per million mapped reads (RPKM).
qRT analysis of LDS and PS transcripts
For OSC expression (PpLDS and PpPS) analysis total RNA was extracted from different adult tissues and early developmental stages of P. parvimensis using TRI-REAGENT (Catalog# T9424, Sigma-Aldrich). RNA samples were treated with Dnase I (Roche). First-strand cDNA synthesis was carried out using SuperScript II Reverse Transcriptase kit according to the manufacturer’s instructions (Invitrogen). Primers used in qRT are listed in Supplementary Table 9.
Whole mount mRNA in situ analysis
Synthesis of PpLDS and PpPS probes
RNA was isolated from 2 dpf and 4 dpf larvae of P. parvimensis using the Total Mammalian RNA Miniprep kit (Sigma-Aldrich) and converted to first strand cDNA with the iScript Select cDNA Synthesis kit (BioRad). Primers were designed against the P. parvimensis sea cucumber OSCs PpLDS and PpPS, such that the PCR products were 500-1000 nt long and the reverse primers had T7 or SP6 tails. The cDNA from the desired stages were used as a template in PCR. Antisense digoxigenin (DIG)-labeled RNA probes were synthesized from PCR products specific to PpLDS or PpPS using the DIG RNA labeling kit (Roche). The primers used are given in Supplementary Table 9.
Whole mount in situ hybridization (WMISH)
WMISH was performed as described earlier with the following modifications54. Pre-hybridization, hybridization and post-hybridization washes were performed at 58°C. Optimal probe concentrations were determined experimentally (~0.2 ng/μl). After blocking, embryos were incubated overnight at 4°C in 2 % blocking reagent (Roche) in malic acid buffer (MAB) with the addition of a 1:2000 dilution of anti-DIG alkaline phosphatase-conjugated antibody (Roche). Embryos were mounted in antifade reagent in glycerol/PBS from the Slowfade antifade kit (Invitrogen) and imaged using Leica DFC420 C camera on a Leica DMI4000B.
Homology modelling
Using human lanosterol synthase (HumanLSS) as a template (pdb: 1w6K)28 homology models of PmLSS, SpLSS, PpLDS and PpPS were developed to identify variations in the active site that govern product specificities of these OSCs. Homology models were generated using the Phyre2 (ver2.0)55 and I-TASSER (ver5.0)56 servers with default parameters. The models obtained were subjected to stereochemical validation using tools embedded in Chimera (ver1.15)57 and visualized using Chimera as well as PyMOL (ver2.0). Protein sequences were aligned using ClustalW (ver2.1) and active site residues were manually annotated using functional information available for human lanosterol synthase28.
Site directed mutagenesis
Site directed mutagenesis was carried to establish whether residue position 444 (F/Q/L) determines product specificity in sea cucumber OSCs. The primer design strategy and PCR conditions used were as described earlier58. Transfer-PCR (TPCR) was used in site-directed mutagenesis reactions59. Briefly, TPCR conditions include: initial denaturation (95°C, 1 min); 13 cycles of 95°C for 30 s, 60°C for 1 min and 72°C for 1.5 min; then 20 cycles of 95°C for 30 s, 67°C for 1 min and 72°C for 4 min. Reactions were completed with a final elongation step of 7 min at 72°C. PCR components include: 5-10 ng of pDONOR or pYES2 plasmid DNA, 20 nM of mutagenic primers, 250 μM of each dNTP, 1x Phusion buffer and 1U of Phusion DNA polymerase (NEB). At the end of the TPCR reaction, 1 μl DpnI was added to 10 μl reaction and incubated at 37°C for 1-2 h. An aliquot (5 μl) of reaction mixture was transformed into E. coli (DH5α, Invitrogen) and positive transformants were verified through sequencing. Primers used in mutagenesis are listed in Supplementary Table 9.
Extended Data
Extended Data Fig. 1. Phylogeny of predicted amino acid sequences of echinoderm OSCs.
Major clades are colored. Candidates with red stars were functionally characterized in yeast in this study. Scale bar 0.1 amino acid substitutions per site. OSCs sequence details are provided in supplementary table 2.
Extended Data Fig. 2. Functional analysis of sea urchin, sea star and sea cucumber OSCs in yeast.
a, Complementation of the LSS-deficient yeast strain (Gil77) with echinoderm OSCs. Yeast transformants spotted undiluted (-), 10- and 100-folds dilution and growth recorded 7 days after spotting. GC-MS total ion chromatograms (TICs) of yeast extracts expressing b, sea star PmLSS and sea urchin SpLSS, c, sea cucumber LDS, d, sea cucumber PS. Superscripts ‘a’ and ‘b’ indicate different accessions of A. japonicus. Peaks marked with asterisks in c and d indicate background modifications of OSC products in yeast. e, GC-MS TICs of yeast extract expressing AjLDSb treated with different concentrations of ketoconazole (CYP51 inhibitor) in the medium (1-50 μg/ml). f-h, GC mass spectra of lanosterol (2), lanostadienol (4) and parkeol (3). pYES2, yeast containing the empty vector (ev). Standards, 2, lanosterol; 4, lanostadienol; 3, parkeol. Ergo = ergosterol, glu = glucose and gal = galactose.
Extended Data Fig. 3. Characterization of sea cucumber saponins.
a, Thin layer chromatography (TLC) of P. parvimenis sea cucumber crude extract and its semi-pure fractions (Fr1-Fr7). b, 96 well yeast growth inhibition (YGI) assay. c, Effect of sea cucumber crude extract and its semi-pure fractions on yeast growth (1-100 μg/ml). Optical density (OD600) was shown as mean ± SD (error bar) with n = 2 replicates. Fr, fraction (see Source Extended Data Fig. 3). d, LC-MS (-) total (TIC) and extracted ion chromatograms (EIC) of fractions Fr5 and Fr6. Saponin peaks of Fr5 and Fr6 are marked with their masses. Fr5; (M-H)- m/z 1391 and m/z 1393, Fr6; (M-H)- m/z 1377 and m/z 1379. e, Q Exactive high resolution LC-MS2 mass fragmentation spectra of saponins observed in fr5 (1391 and 1393) and fr6 (1377 and 1379), their deduced saponin structures showing nature of fragmentation (blue lines and red numbers) and high-resolution masses of parent and product ions etc. Glc; glucose, MeGlc; methyl glucose, Qui; quinovose, Xyl; xylose, Ag; aglycone. Aglycone mass is without the δ lactone moiety because it is lost as CO2 (M-H-CO2). Structural details on saponin sugar chains are given in Supplementary Fig. 4.
Extended Data Fig. 4. LC-MS (-) analysis of sea cucumber tissue extracts.
a, LC-MS (-) TICs of sea cucumber adult tissue extracts and b, early stages. All tissue wise replicate profiles are shown. Aj, A. japonicus n = 3; Pp, P. parvimensis n = 2. Rt; retention time. Saponin peaks with same retention times and masses are connected by a dotted line and annotated with compound names or their masses. c-d, Q Exactive high resolution LC-MS analysis showing detection of saponin ions in A. japonicus (Aj) and P. parvimensis (Pp) early-stage extracts. Saponin ions highlighted in bold were subjected for LC-MS2 and its corresponding data shown in Extended Data Fig. 5b.
Extended Data Fig. 5. Antifungal activity, saponin content and OSC gene expression in sea cucumber juvenile stages.
a, Top, inhibition of yeast growth by crude extracts of early growth stages of A. japonicus and P. parvimensis. Methanol (MeOH) used as a control (mean ± SD, n = 2) (see Source Extended Data Fig. 5). Middle, presence (+) or absence (-) of saponins in P. parvimensis (Pp) and A. japonicus (Aj) based on LC-MS profiles shown in Extended Data Fig. 4b-d. Bottom, heat map showing low (green) to high (red) OSC gene expression generated from RPKM values (Aj, n = 3). b, Q Exactive high resolution LC-MS2 mass fragmentation spectra of saponins observed in early-stage extracts, their deduced saponin structures and high-resolution masses of parent and product ions observed. Glc; glucose, MeGlc; methyl glucose, Qui; quinovose, Xyl; xylose, Ag; aglycone. Structural details on saponin sugar chains are given in Supplementary Fig. 4.
Extended Data Fig. 6. Sea stars and sea cucumbers make unusual sterols.
a, Representative GC-MS profiles (TICs) of sea urchin, sea star and sea cucumber adult tissues. The peaks at 10.1, 10.4 and 11.52 min are lathosterol (7), episterol (22), and avenasterol (21), respectively. Peak eluting at 9.98 min unique to sea cucumbers and was characterized to be 14α-methylcholest-9(11)-en-3β-ol (11) (Supplementary Table 5-7). b, Levels of cholesterol (5), lathosterol (7) and 14α-methylcholest-9(11)-en-3β-ol (11) in adult tissues of A. japonicus (mean ± SD, n = 2). Dw, dry weight (see Source Extended Data Fig. 6). c, Unusual sterols renders sea star and sea cucumber membranes saponin resistant. 3D conformations of cholesterol (5), lathosterol (7) and 14α-methylcholest-9(11)-en-3β-ol (11) showing flat conformation of side chain in 5 and bent conformation in 7 and 11. 3D conformations were optimized using Frog2 server with default parameters60. d, GC mass spectrum of the sterols observed. Avenasterol (21) and episterol (22) and were identified based on known spectra in the literature and others based on authentic standards. All sterols are TMS derivatives.
Extended Data Fig. 7. Sea stars and sea cucumbers evolved unusual sterol pathways.
a, Canonical cholesterol biosynthetic pathway of animals including sea urchins and sea stars. b, Sea star and sea cucumber specific biosynthetic pathway of Δ7 sterols, lathosterol (7), avenasterol (21) and episterol (22). Sea stars make lathosterol (7) from cholesterol (5) that is made de novo whereas sea cucumbers make lathosterol (7) through diet derived cholesterol (5) or other Δ5 sterols. c,Neighbor-joining tree of C7Ds (cholesterol 7-desaturse) hits from echinoderms and other functionally characterized sequences. Sequence names are uniprot sequence identifiers. d, Sequence alignment of C7D/DAF36 active site motifs of echinoderm as well as other functionally characterized sequences. Residue positions marked with red arrows are part of the Rieske motif which co-ordinates iron and sulfur required in cholesterol 7-desaturation.
Extended Data Fig. 8. A single active site residue (position 444) determines cyclization mechanism and product specificity of LSS, LDS and PS OSCs.
a, The cyclization mechanism for lanosterol (2), lanostadienol (4), parkeol (3) and cycloartenol. Protonation, cyclization, and rearrangement of 2, 3 oxidosqualene (1) to a central protosteryl cation (C20). Rearrangement of C20 cation lead to either C8 or C9 cations depending on type of OSC involved. Deprotonation at C7 of C8 cation lead to lanostadienol (4) and deprotonation at C11 of C9 cation lead to parkeol (3). Lanosterol (2) and cycloartenol are derived from C8 and C9 cations, respectively. b, OSC sequence alignment of 24 active site residue positions of echinoderms and others (sequences given in supplementary notes 2). c, OSC active site region is shown in yellow and region encompassing 5Å around lanosterol is in blue. d, GC-MS TICs of yeast extracts expressing AjPSa-WT and its mutants AjPSa-L436F, AjPSa-L436Q. Peaks marked with asterisks are undesired modifications of the PS product in yeast. e-f, TICs and EICs (m/z 426) of yeast extracts expressing SpLSS-WT and its mutants SpLSS-F440L, SpLSS-F440Q. Standards: 2, lanosterol; 4, lanostadienol; 3, parkeol.
Extended Data Fig. 9. Discovery of Gemmata obscuriglobus parkeol synthase (GoPS).
a, OSCs sequence alignment of residue position 444 from diverse taxa. Bacterial group-I OSCs with variation at residue position 444 are shown in green. Sequences used in phylogeny and sequence alignment are given in supplementary notes 2. b, Neighbor-joining tree of representative OSCs from diverse taxa. Holozoan clade (blue arc) includes all animal representatives. Bacterial squalene hopane cyclases (SHC) used as outgroups. Bacterial group-I OSCs with ‘L’ natural mutation (yellow). Bootstrap percentages higher than 50 are shown (500 replicates). c, GC-MS TICs of yeast extract expressing GoPS. Peaks marked with asterisks are undesired modifications of the PS product in yeast. Standards, 2, lanosterol; 4, lanostadienol; 3, parkeol.
Extended Data Fig. 10. Model for evolutionary origin of saponins and unusual sterols in sea stars and sea cucumbers.
a, Phylogeny of sea stars, sea urchins and sea cucumbers based on ref16. b, Genomic neighborhood of OSC genes. OSC genes are shown in different shades of green, and CYP51 genes in orange. Red cross in CYP51 denote gene loss. Colored stars denote OSC mutations at amino acid residue position 444. Scaffolds and gene modules are not to scale. c, Presence, or absence of saponins in sea urchins, sea stars and sea cucumbers. d, Major sterols of sea urchins, sea stars and sea cucumbers.
Supplementary Material
Acknowledgments
We thank Peter J. Bryant (University of California, Irvine) for permission to use the sea cucumber image shown in Fig. 1a, Hans-Wilhelm Nützmann (John Innes Centre; now at the University of Bath) for assistance with P. parvimensis qRT, Zheyong Xue (Northeast Forestry University, Harbin, China) for providing samples of dried A. japonicus, Damien P. Devos (CABD, Spain) for providing G. obscuriglobus genomic DNA, Gary M. Wessel (Brown University, US) for P. miniata RNA samples, and Lionel Hill and Paul Brett (John Innes Centre Metabolite Services) for advice on metabolite analysis. We also thank Michael J. Stephenson (John Innes Centre) for critical reading of the draft manuscript. A.O. and R.T. acknowledge funding support provided by European Union grant KBBE-2013-7 (TriForC), the Biotechnological and Biological Sciences Research Council Institute Strategic Programme Grant ‘Molecules from Nature -Products and Pathways’ (BBS/E/J/000PR9790) and the John Innes Foundation. R.T also acknowledges funding support from DBT-Ramalingaswami fellowship, India. S.W. and Z.B. acknowledge the support of the National Natural Science Foundation of China (32130107). the Taishan Scholar Project Fund of Shandong Province of China and the Sanya Yazhouwan Science and Technology City Management Foundation (SKJC-KJ-2019KY01).
Footnotes
Author contributions. R. Thimmappa carried out bioinformatics, gene cloning, vector construction, analysis of wild type and mutant variants of OSCs, saponin and sterol extraction, quantification, analysis and purification; A. Huang performed NMR analysis and structural assignments; R. Chandra Misra carried out the mutational analysis of SpLSS in yeast and part of the saponin analysis using LC-MS2; G. Saalbach carried out initial MALDI-Tof analysis; M. Zheng and V. Hinman provided sea cucumber, sea star and sea urchin tissues and cDNA and carried out mRNA in situ analysis of sea cucumber OSCs; S. Wang, Z. Bao, Y. Chang and Z. Zhou provided sea cucumber LAS sequences, access to previously published transcriptome data, and samples of sea cucumber tissues for saponin and sterol analysis. All authors contributed to the preparation of the manuscript. R. Thimmappa and A. Osbourn conceived the project and wrote the paper.
Competing interest declaration. The authors declare no competing interests.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this article is available as a Supplementary Information file. The datasets, constructs and chemical standards generated and analysed during the current study are available from the corresponding author upon request. Sea cucumber material is available from co-authors Veronica Hinman (Carnegie Mellon University, Pittsburgh, US; email: veronica@cmu.edu) and Shi Wang (Ocean University of China, Qingdao, China; email: swang@ouc.edu.cn). All associated raw data is provided in Supplementary Data 1 to 7. The sequences of genes characterized within this project will be deposited in the European Nucleotide Archive and accession numbers will be shared before publication.
References
- 1.Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25(12):640–644. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Shimada S. Antifungal steroid glycoside from sea cucumber. Science. 1969;163(3874):1462. doi: 10.1126/science.163.3874.1462. [DOI] [PubMed] [Google Scholar]
- 3.Minale L, Pizza C, Riccio R, Zollo F. Steroidal glycosides from starfishes. Pure & Appl Chem. 1982;54(10):1935–1950. [Google Scholar]
- 4.Yasumoto T, Tanaka M, Hashimoto Y. Distribution of saponin in echinoderms. Bull Japan Soc Sci Fish. 1966;32(8):673–676. [Google Scholar]
- 5.Dyck SV, et al. The triterpene glycosides of Holothuria forskali: Usefulness and efficiency as a chemical defense mechanism against predatory fish. J Exp Biol. 2011;214:1347–1356. doi: 10.1242/jeb.050930. [DOI] [PubMed] [Google Scholar]
- 6.Kalinin VI, et al. In: Echinoderm Studies. Jangoux M, Lawrence JM, editors. 5A.A. Balkema; Rotterdam, Netherland: 1996. Biological activities and biological role of triterpene glycosides from holothhuroids (Echinodermata) pp. 139–181. [Google Scholar]
- 7.Hamel J, Mercier A. Evidence of chemical communication during the gametogenesis of Holothuroids. Ecology. 1996;77:1600–1616. [Google Scholar]
- 8.Naruse M, et al. Acrosome reaction-related steroidal saponin, Co-ARIS, from the starfish induces structural changes in microdomains. Dev Biol. 2010;347:147–153. doi: 10.1016/j.ydbio.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Caulier G, Flammang P, Gerbaux P, Eeckhaut PI. When a repellent becomes an attractant: harmful saponins are kairomones attracting the symbiotic Harlequin crab. Sci Rep. 2013;3:2639. doi: 10.1038/srep02639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flórez LV, Biedermann PH, Engl T, Kaltenpoth M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep. 2015;32(7):904–936. doi: 10.1039/c5np00010f. [DOI] [PubMed] [Google Scholar]
- 11.Claereboudt EJS, Eeckhaut I, Lins L, Deleu M. How different sterols contribute to saponin tolerant plasma membranes in sea cucumbers. Sci Rep. 2018;8(1):10845. doi: 10.1038/s41598-018-29223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods - A Review. Marine Drugs. 2011;9(10):1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thimmappa R, Geisler K, Louveau T, O’Maille P, Osbourn A. Triterpene biosynthesis in plants. Annu Rev Plant Biol. 2014;65:225–2014. doi: 10.1146/annurev-arplant-050312-120229. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, et al. Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discov. 2018;26:4–29. doi: 10.1038/s41421-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall MR, et al. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature. 2017;544:231–234. doi: 10.1038/nature22033. [DOI] [PubMed] [Google Scholar]
- 16.Kudtarkar P, Cameron RA. Echinobase: an expanding resource for echinoderm genomic information. Database. 2017;2017:1–9. doi: 10.1093/database/bax074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, et al. The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 2017;15(10):e2003790. doi: 10.1371/journal.pbio.2003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushiro T, Shibuya M, Ebizuka Y. β-Amyrin synthase-cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur J Biochem. 1998;256(1):238–244. doi: 10.1046/j.1432-1327.1998.2560238.x. [DOI] [PubMed] [Google Scholar]
- 19.Ito R, et al. Triterpene cyclases from Oryza sativa L.: cycloartenol, parkeol and achilleol B synthases. Org Lett. 2011;13(10):2678–2681. doi: 10.1021/ol200777d. [DOI] [PubMed] [Google Scholar]
- 20.Iniguez-Martinez, Guerra-Rivas G, Rios L, Quijano T. Triterpenoid oligoglycosides from the sea cucumber Stichopus parvimensis. J Nat Prod. 2005;68:1669–1673. doi: 10.1021/np050196m. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, et al. Antifungal nortriterpene and triterpene glycosides from the sea cucumber Apostichopus japonicus Selenka. Food Chemistry. 2012;132(1):295–300. doi: 10.1016/j.foodchem.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 22.Pivkin MV. Filamentous fungi associated with holothurians from the sea of Japan, off the primorye coast of Russia. Biol Bull. 2000;198(1):101–109. doi: 10.2307/1542808. [DOI] [PubMed] [Google Scholar]
- 23.Iyengar EV, Harvell CD. Predator deterrence of early developmental stages of temperate lecithotrophic asteroids and holothuroids. J Exp Mar Bio Ecol. 2001;264(2):171–188. [Google Scholar]
- 24.Anisimov MM, Fronert EB, Kuznetsova TA, Elyakov GB. The toxic effects of triterpene glycosides from Stichopus japonicus on early embryogenesis of the sea urchin. Toxicon. 1973;11(1):109–111. doi: 10.1016/0041-0101(73)90163-3. [DOI] [PubMed] [Google Scholar]
- 25.Fusetani N, Kato Y, Hashimoto K. Biological effects of asterosaponins with special reference to structure-activity relationships. J Nat Prod. 1984;47(6):997–1002. [Google Scholar]
- 26.Stonik VA, et al. Free sterol compositions from the sea cucumbers Pseudostichopus trachus, Holothuria nobilis, Holothuria scabra, Trochostoma orientale and Bathyplotes natans. Comp Biochem Physiol. 1998;120B:337–347. [Google Scholar]
- 27.Wollam J, et al. The Rieske oxygenase DAF-36 functions as a cholesterol 7-desaturase in steroidogenic pathways governing longevity. Aging Cell. 2011;10(5):879–884. doi: 10.1111/j.1474-9726.2011.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoma R, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature. 2004;432(7013):118–122. doi: 10.1038/nature02993. [DOI] [PubMed] [Google Scholar]
- 29.The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepesheva GI, Waterman MR. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochimica et Biophysica Acta. 2007;1770(3):467–477. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCauley BS, Wright EP, Exner C, Kitazawa C, Hinman VF. Development of an embryonic skeletogenic mesenchyme lineage in a sea cucumber reveals the trajectory of change for the evolution of novel structures in echinoderms. EvoDevo. 2012;3(1):17. doi: 10.1186/2041-9139-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang QL, Liu YH. Cultivation techniques on sea cucumber and sea urchin. Ocean University of China Press; 1998. [Google Scholar]
- 33.Reich A, Dunn C, Akasaka K, Wessel G. Phylogenomic Analyses of Echinodermata Support the Sister Groups of Asterozoa and Echinozoa. PloS ONE. 2015;10(3):e0119627. doi: 10.1371/journal.pone.0119627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janies DA, et al. EchinoDB, an application for comparative transcriptomics of deeply sampled clades of echinoderms. BMC Bioinformatics. 2016 doi: 10.1186/s12859-016-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solovyev V, Kosarev P, Seledsov I, Vorobyev D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006 doi: 10.1186/gb-2006-7-s1-s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Racolta S, Juhl PB, Sirim J, Pleiss D. The triterpene cyclase protein family: a systematic analysis. Proteins. 2012;80:2009–2019. doi: 10.1002/prot.24089. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 39.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Mizutani K. High-throughput plasmid construction using homologous recombination in yeast: its mechanisms and application to protein production for X-ray crystallography. Biosci Biotechnol Biochem. 2015;79(1):1–10. doi: 10.1080/09168451.2014.952614. [DOI] [PubMed] [Google Scholar]
- 41.Takase S, et al. Control of the 1,2-rearrangement process by oxidosqualene cyclases during triterpene biosynthesis. Org Biomol Chem. 2015;13(26):7331–7336. doi: 10.1039/c5ob00714c. [DOI] [PubMed] [Google Scholar]
- 42.Herrera JBR, Wilson WK, Matsuda SPT. Tyrosine-to-threonine mutation converts cycloartenol synthase to an oxidosqualene cyclase that forms lanosterol as its major product. J Am Chem Soc. 2000;122(28):6765–6766. [Google Scholar]
- 43.Turner EM. The nature of resistance of oats to the take-all fungus. III. Distribution of the inhibitor in oat seedlings. J Exp Bot. 1960;11:403–412. [Google Scholar]
- 44.Kalinovskaya NI, Kuznetsova TA, Elyakov GB. Sterol composition of Pacific holothurian Stichopus japonicus selenka. Comp Biochem Physiol B. 1983;74(3):597–601. [Google Scholar]
- 45.Itoh T, Ishii T, Tamura T, Matsumoto T. Four new and other 4α-methylsterols in the seeds of Solanaceae. Phytochemistry. 1978;17:971–1978. [Google Scholar]
- 46.Makarieva TN, et al. Biosynthetic studies of marine lipids. 42. Biosynthesis of steroid and triterpenoid metabolites in the sea cucumber Eupentacta fraudatrix. Steroids. 1993;58:508–517. doi: 10.1016/0039-128x(93)90026-j. [DOI] [PubMed] [Google Scholar]
- 47.Goad LJ, Garneau FX, Simard JL, ApSimon JW, Girard M. Isolation of Δ9(11) sterols from the sea cucumber Psolus fabricii Implication for holothurin biosynthesis. Tetrahedron Lett. 1985;26:3513–3517. [Google Scholar]
- 48.Goad LJ, Garneau FX, Simard JL, ApSimon JW, Girard M. Composition of the free, esterified and sulphated sterols of the sea cucumber Psolus fabricii. Comp Biochem Physiol B. 1986;84:189–196. [Google Scholar]
- 49.Cordeiro ML, Kerr RG, Djerassi C. Conversion of parkeol (lanosta-9(11),24-dien-3β-ol) to 14α-methylcholest-9(11)-en-3β-ol in the sea cucumber Holothuria haracter. Tetrahedron Lett. 1988;29:2159–1988. [Google Scholar]
- 50.Cordeiro ML, Djerassi C. Biosynthetic studies of marine lipids. 25. Biosynthesis of Δ9(11)- and Δ7(8)-sterols and saponins in sea cucumbers. J Org Chem. 1990;55:2806–1990. [Google Scholar]
- 51.Du H, et al. Transcriptome sequencing and characterization for the sea cucumber Apostichopus japonicus (Selenka, 1867) PloS ONE. 2012;7:e33311. doi: 10.1371/journal.pone.0033311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinman VF, Nguyen AT, Davidson EH. Expression and function of a starfish Otx ortholog, AmOtx: a conserved role for Otx proteins in endoderm development that predates divergence of the eleutherozoa. MOD. 2003;120(10):1165–1176. doi: 10.1016/j.mod.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Kelley LA, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettersen EF, et al. UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 58.Salmon M, et al. A conserved amino acid residue critical for product and substrate specificity in plant triterpene synthases. Proc Natl Acad Sci U S A. 2016;113(30):E4407–E4414. doi: 10.1073/pnas.1605509113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erijman A, Dantes A, Bernheim R, Shifman JM, Peleg Y. Transfer-PCR (TPCR): A highway for DNA cloning and protein engineering. J Struct Biol. 2011;175(2):171–177. doi: 10.1016/j.jsb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Miteva MA, Guyon F, Tufféry P. Frog2: Efficient 3D conformation ensemble generator for small compounds. Nucleic Acids Res. 2010;38:W622–627. doi: 10.1093/nar/gkq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this article is available as a Supplementary Information file. The datasets, constructs and chemical standards generated and analysed during the current study are available from the corresponding author upon request. Sea cucumber material is available from co-authors Veronica Hinman (Carnegie Mellon University, Pittsburgh, US; email: veronica@cmu.edu) and Shi Wang (Ocean University of China, Qingdao, China; email: swang@ouc.edu.cn). All associated raw data is provided in Supplementary Data 1 to 7. The sequences of genes characterized within this project will be deposited in the European Nucleotide Archive and accession numbers will be shared before publication.