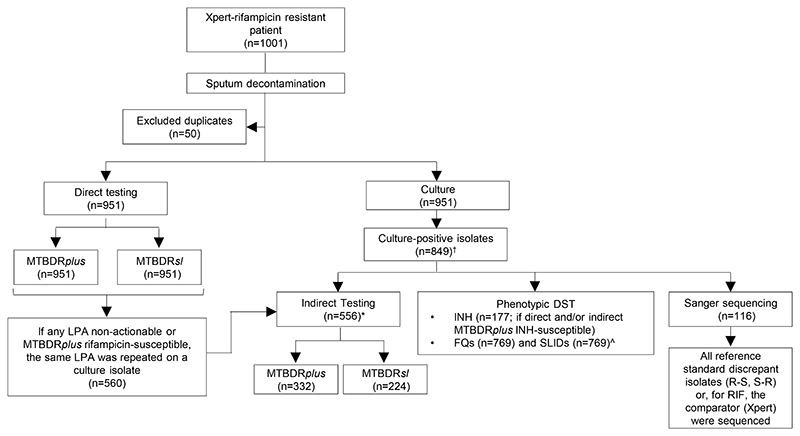
Figure 1.
Testing flow diagram showing direct and indirect testing using MTBDRplus and MTBDRsl and the use of reference standard phenotypic testing for second-line drugs, irrespective of the LPA result. Prior to the study, the flow of tests was the same except MTBDRsl was not used and MTBDRplus was only done directly if the specimen was smear-positive.
*4 direct non-actionables were culture-negative and unable to be test indirectly
†102 Xpert-positives were not culture-positive and hence did not have an isolate available ^80 isolates were contaminated upon regrowth for FQ and SLID pDST
Abbreviations: DST-drug susceptibility testing, R-resistant, S-susceptible, n-number, INH-isoniazid, MDR-multi drug resistant, Xpert-Xpert MTB/RIF, FQs-fluoroquinolones, SLID-second-line injectables drugs, n-number, LPA-line probe assay.