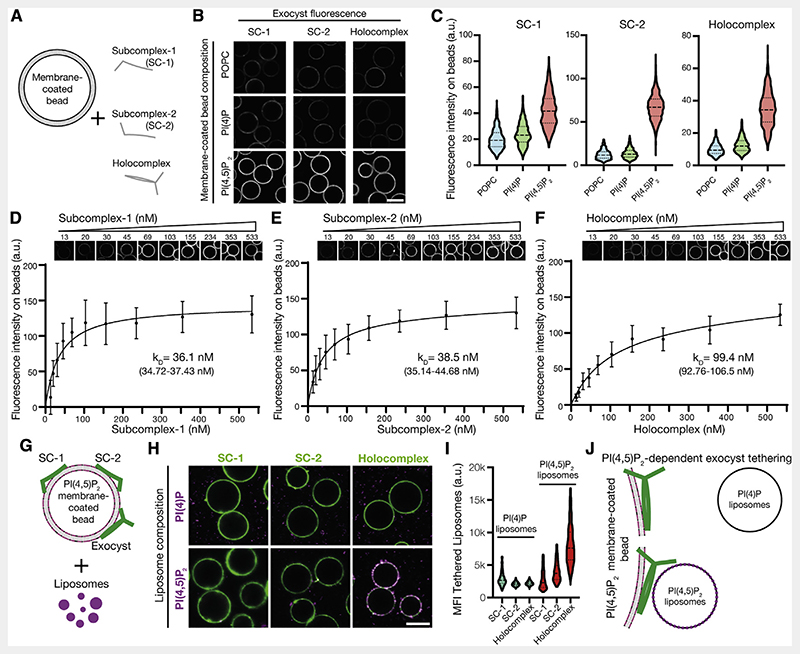
Figure 2. Independent binding of exocyst subcomplexes-1 and -2 to PI(4,5)P2 enables holocomplex tethering.
(A) Schematic of phosphoinositide specificity experiment. Membrane-coated beads of distinct lipid composition were generated. To these beads, fluorescently tagged exocyst subcomplexes were added to assess membrane binding by fluorescence microscopy.
(B) Reconstituted fluorescently tagged subcomplexes-1 and -2 or holocomplex were added at 100 nM to membrane-coated beads. These were formed from a lipid composition of 84.9% 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), 10% phosphatidylserine, and 5% phosphoinositide or POPC, doped with 0.1% rhodamine-DPPE. Beads were imaged by confocal microscopy. Scale bar, 10 μm.
(C) Membrane-coated beads were segmented using the rhodamine lipid signal as a membrane mask, and the mean fluorescence intensities of exocyst complexes bound to each bead were determined. Violin plots, 877–1,395 segmented beads per condition.
(D–F) Relative PI(4,5)P2 membrane binding affinities for the exocyst complexes. Subcomplexes-1 and -2 or holocomplex was added in a dilution series to membrane-coated beads containing 5% PI(4,5)P2 and imaged by confocal microscopy. Individual beads were segmented, and a binding saturation curve was fitted resulting in mean kD determination with 95% confidence intervals given. In total, 340–1,359 beads were quantified per concentration, mean ± standard deviation.
(G) Schematic of liposome tethering experiment. Subcomplexes-1 and -2 or holocomplex (150 nM) were bound to 5% PI(4,5)P2 membranes. Liposomes doped with Atto647N-DOPE and composed with either 5% PI(4)P or PI(4,5)P2 were added.
(H and I) Membranes and exocyst complexes were imaged by confocal microscopy, segmented, and the fluorescent intensity of tethered liposomes was measured. Violin plots from 430–778 individual beads. MFI, mean fluorescent intensity. Scale bar, 10 μm.
(J) Exocyst holocomplex tethers two PI(4,5)P2, but not PI(4)P, containing membranes to each other.
See also Figure S2.