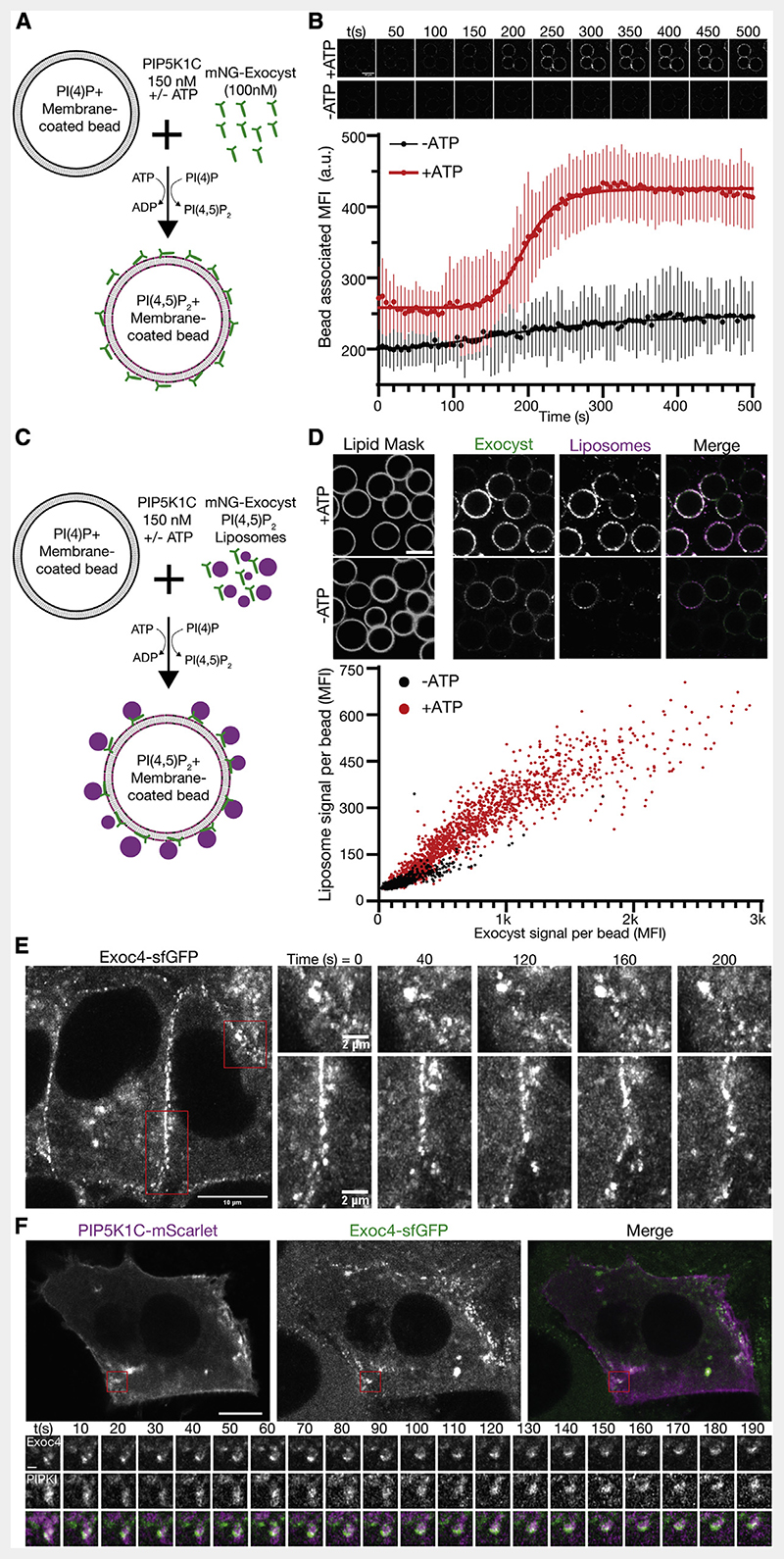
Figure 4. Phosphoinositide conversion drives exocyst-mediated membrane tethering.
(A) Schematic of exocyst recruitment by phosphoinositide conversion. Membrane-coated beads containing PI(4)P were mixed with exocyst (100 nM) and PIP5K1C (150 nM) in the presence and absence of ATP.
(B) Exocyst is recruited to membrane-coated beads upon ATP-dependent PI(4)P to PI(4,5)P2 conversion, and imaged by confocal microscopy. Beads were segmented, and the associated exocyst mean fluorescent intensity was measured over time. Mean ± standard deviation, n = 44, 68 beads. Scale bar, 10 μm.
(C) Schematic of membrane tethering by phosphoinositide conversion. PI(4,5)P2 containing liposomes were added to the phosphoinositide conversion experiment.
(D) The intensity of bound exocyst and tethered liposomes was determined after 30 min, and the mean fluorescent intensity of each was plotted per bead. n = 1,440, 1,864 beads for —ATP and +ATP, respectively. Scale bar, 10 μm.
(E) NMuMG cells with endogenous Exoc4-sfGFP were imaged by live-cell Airyscan microscopy. Insets show the dynamics of plasma membrane-associated exocyst subdomains.
(F) Cells were transfected with mScarlet-tagged PIP5K1C and imaged by live-cell Airyscan microscopy. Inset shows time series of exocyst colocalization with PIP5K1C at dynamic subdomains of the plasma membrane. Scale bar, 10 μm, inset scale bar, 1 μm.