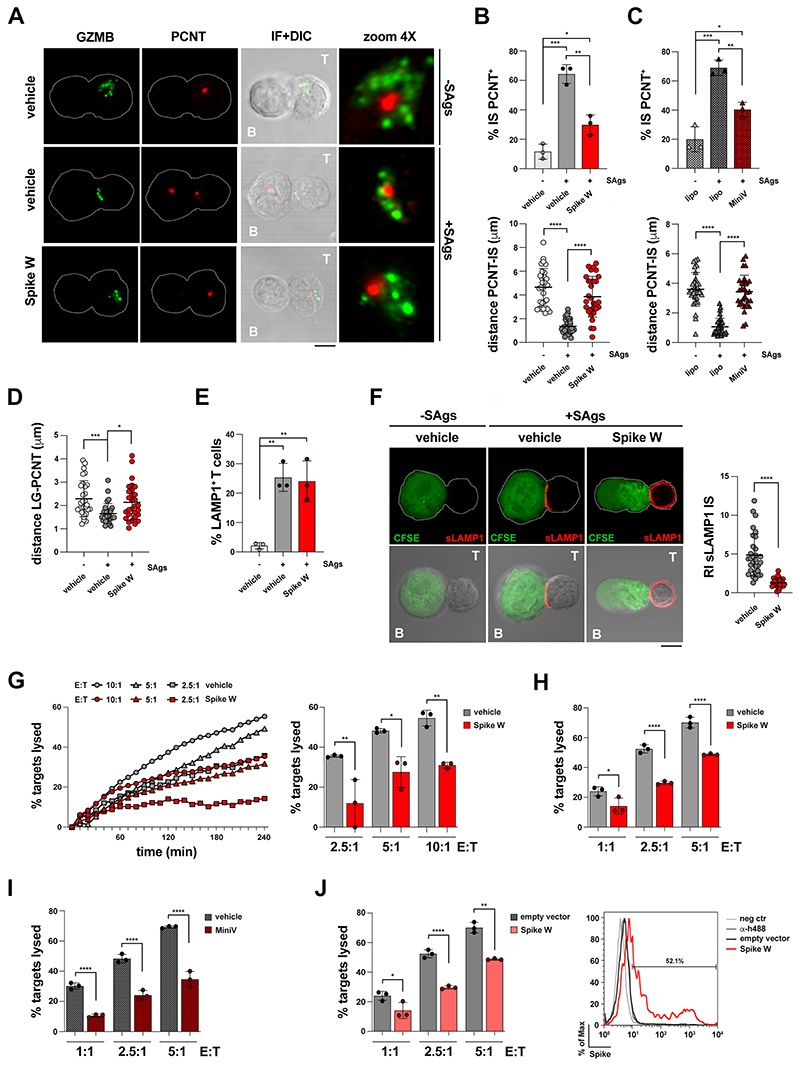
Figure 3. Spike inhibits centrosome and lytic granule polarisation to the CTL IS and CTL-mediated cytotoxicity.
A-D. Immunofluorescence analysis of PCNT and GrzmB in CTLs (day 7) pre-treated with either vehicle (PBS) or 0.05 μg/μl Spike Wuhan (Spike W) (A,B,D), or with 1.2×109 control liposomes or MiniVs (A-C), then mixed with Raji cells (APCs) either unpulsed or pulsed with a combination of SEA, SEB and SEE (SAgs), and incubated for 15 min at 37°C. Representative images (medial optical sections) of the conjugates are shown (A). B,C. Top, Quantification (%) of 15-min SAg-specific conjugates harboring PCNT staining at the IS (≥50 cells/sample, n=3, One-way ANOVA test, *** p≤0.001; **p≤0.01; *p≤0.05). Bottom, Measurement of the distance (μm) of the centrosome (PCNT) from the CTL-APC contact site (10 cells/sample, n=3, One-way ANOVA test, ****p≤0.0001). D. Measurement of the distance (μm) of the lytic granules (LG, marked by GzmB) from the centrosome (PCNT) in conjugates formed with Spike W-pre-treated CTLs (10 cells/sample, n=3, Kruskal-Wallis test, *** p≤0.001; *p≤0.05) (see figure S2 for parameters used for measurements plotted in panels B-D). E. Flow cytometry analysis of degranulation of CTLs (day 7) pre-treated with either vehicle (PBS) or Spike Wuhan (Spike W) and co-cultured with CFSE-stained Raji cells loaded with SAg at an E:T cell ratio 2.5:1 for 1 h. The histogram shows the percentage (%) of LAMP1+ T cells (n=3, One-way ANOVA test, **p≤0.01). F. Immunofluorescence analysis of degranulation of CTLs (day 7) pre-treated with either vehicle (PBS) or Spike Wuhan (Spike W) and co-cultured with CFSE-stained Raji cells loaded with SAg at an E:T cell ratio 2.5:1 for 1 h. Conjugates were fixed and stained without prior permeabilization with anti-LAMP1 mAb and fluorescently labelled secondary Abs to detect plasma membrane-associated LAMP1. The histogram shows the relative surface LAMP1 fluorescence (sLAMP1) intensity at the IS (recruitment index) of SAg-specific conjugates in the absence or presence of Spike (10 cells/sample, n=3, Kruskal-Wallis test, ****p≤0.0001). Negative control conjugates (no SAg) were not quantified as no sLAMP1 fluorescence was detectable. Representative images (medial optical sections) of the conjugates are shown. G. Fluorimetric analysis of cytotoxicity of CTLs (day 7) using the calcein release assay. CTLs were pre-treated with either vehicle (PBS) or Spike Wuhan (Spike W) and co-cultured with SAg-pulsed, calcein AM-loaded Raji cells at different E:T cell ratios for 4 h. The representative curves show the kinetics of target cell lysis by CTLs at the indicated E:T cell ratios. The histogram shows the target cell lysis at 4 h (n=3, One-way ANOVA test, **p≤0.01; *p≤0.05). H,I. Flow cytometric analysis of antigen-specific target cell killing by melanoma-specific CTLs derived from 3 patients, pre-treated with either vehicle (PBS) or Spike Wuhan (Spike W), or with 1.2×109 control liposomes or MiniVs, using as target the melanoma cell line A375. Cells were co-cultured for 18 h and stained with propidium iodide and anti-CD8 mAb prior to processing for flow cytometry. Analyses were carried out gating on CD8-/PI+ cells. The histograms show the percentage (%) of target cells lysed (n=3, One-way ANOVA test, ****p≤0.0001; *p≤0.05). J. Flow cytometric analysis of antigen-specific target cell killing by melanoma-specific CTLs derived from 3 patients, using as target A375 melanoma cells transiently transfected with either a construct encoding Spike-W or the empty control vector (54.0±1.7 % transfected cells, as assessed by flow cytometric analysis with anti-Spike mAb J08; representative flow cytometric data are shown). Cells were co-cultured for 18 h and processed as in (I). The histograms show the percentage (%) of target cells lysed (n=3, One-way ANOVA test, ****p≤0.0001; **p≤0.01; *p≤0.05). Data are expressed as mean±SD. Size bar, 5 μm.. Non-significant differences are not shown.