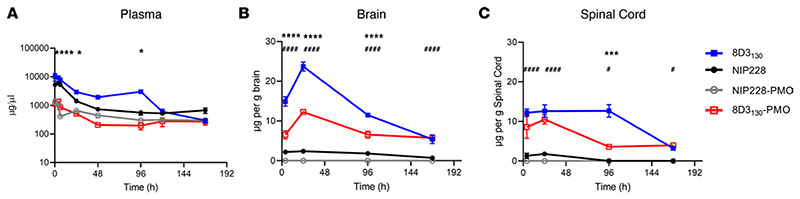
Figure 2. PK of antibody and antibody-PMO conjugates in mice.
Plasma, brain, and spinal cord exposure following 20 mg/kg dose of 8D3130 (±PMO) and NIP228 (±PMO), unconjugated and conjugated to the PMO. (A) Plasma PK of antibodies with or without PMO over a 1-week period. High statistical significance (****) shown for 8D3130 20 mg/kg versus 8D3130-PMO 20 mg/kg for the first 3 time points and a lower significance (*) for 24- and 96- hour time points. No statistical significance shown for NIP228-PMO 20 mg/kg versus 8D3130-PMO 20 mg/kg at any time point. (B) Brain exposure as a measure of μg compound/g brain. Statistical significance (****) shown for 8D3130 20 mg/kg versus 8D3130-PMO 20 mg/kg for first 3 time points. Statistical significance (####) shown for NIP228-PMO 20 mg/kg versus 8D3130-PMO 20 mg/kg at all time points. (C) Spinal cord exposure as a measure of μg compound/g spinal cord. Statistical significance (***) shown for 8D3130 20 mg/kg versus 8D3130-PMO 20 mg/kg at 96-hour time point. Statistical significance (####) shown for NIP228-PMO 20 mg/kg versus 8D3130-PMO 20 mg/kg at first 2 time points and a lower statistical significance (#) at the last 2 time points. Statistical significance (representative P values) for exposure in brain, spinal cord, and plasma between 8D3130 20 mg/kg versus 8D3130-PMO 20 mg/kg (*) and NIP228-PMO 20 mg/kg versus 8D3130-PMO 20 mg/kg (#) at all time points evaluated. Statistical analysis was performed in GraphPad Prism. Data shown as the mean ± SEM, n = 3−4 per group. Statistical significance shown using 2-way ANOVA, where appropriate, made using Tukey test. *P, < 0.05; ***P, < 0.001; ****P, < 0.0001; #P, < 0.05; ####P, < 0.0001.