Abstract
Type 2-high asthma is a chronic inflammatory disease of the airways which is increasingly prevalent in countries where helminth parasite infections are rare, and characterized by T helper 2 (Th2)-dependent accumulation of eosinophils in the lungs. Regulatory cytokines such as TGF-β can restrain inflammatory reactions, dampen allergic Th2 responses, and control eosinophil activation. The murine helminth parasite Heligmosomoides polygyrus releases a TGF-β mimic (Hp-TGM) that replicates the biological and functional properties of TGF-β despite bearing no structural similarity to the mammalian protein. Here, we investigated if Hp-TGM could alleviate allergic airway inflammation in mice exposed to Alternaria alternata allergen, house dust mite (HDM) extract or alum-adjuvanted ovalbumin protein (OVA). Intranasal administration of Hp-TGM during Alternaria exposure sharply reduced airway and lung tissue eosinophilia along with bronchoalveolar lavage fluid IL-5 and lung IL-33 cytokine levels at 24 h. The protective effect of Hp-TGM on airway eosinophilia was also obtained in the longer T-cell mediated models of HDM or OVA sensitisation with significant inhibition of eotaxin-1, IL-4 and IL-13 responses depending on the model and timepoint. Hp-TGM was also protective when administered parenterally either when given at the time of allergic sensitisation or during airway allergen challenge. This project has taken the first steps in identifying the role of Hp-TGM in allergic asthma and highlighted its ability to control lung inflammation and allergic pathology. Future research will investigate the mode of action of Hp-TGM against airway allergic eosinophilia, and further explore its potential to be developed as a biotherapeutic in allergic asthma.
Keywords: airway allergy, cytokines, eosinophils, helminth immunomodulators
Introduction
Type 2-high asthma is a chronic inflammatory disease of the airways leading to cough, wheeze, shortness of breath, and chest tightness. This form of asthma is induced by early-life encounters with environmental allergens, including house dust mite (HDM) and Alternaria alternata, which evoke T helper 2 (Th2) cell responses [1]. Exuberant production of IL-4, IL-5, and IL-13 leads to asthma features with the accumulation of type 2-associated cells, such as eosinophils, in lung tissue [2, 3–5]. Eosinophil infiltration into the lung plays a causal role in augmentation of broader inflammation and is associated with asthma disease severity [1, 6, 7]. Targeting eosinophils has shown promise in alleviating asthma in clinical trials with therapeutic antibodies blocking type 2 cytokines or their receptors [3, 4, 8–14].
Eosinophils are regulated by a suite of cells, cytokines, and chemokines. In response to inhaled allergens, alarmin cytokines such as IL-33 are released by necrotic airway epithelial cell, triggering type 2 dendritic cells, which migrate from the lung tissue to the draining lymph node to induce Th2 differentiation [15]. Type 2 innate lymphoid cells (ILC2s) also respond to IL-33, and together with Th2 cells produce interleukin (IL)-5, to induce eosinophil differentiation, activation and recruitment into inflamed lungs [16]. Contemporaneously, IL-4 and IL-13 production stimulates the generation of eotaxins (e.g., Eotaxin-1/CCL-11), the main chemoattractants for eosinophils produced by lung epithelial cells, fibroblasts and smooth muscle cells [4, 17].
IL-5 is considered to be the major cytokine in eosinophil biology, mobilizing eosinophils from the bone marrow during allergic inflammation, and driving their activation and proliferation in peripheral tissues [1, 13, 18]. Together with IL-3 and granulocyte/macrophage colony-stimulating factor (GM-CSF), IL-5 can prolong eosinophil survival and abrogate their apoptosis [19]. In addition, specific subsets of dendritic cells (type 1 dendritic cells) attract eosinophils into the airways in asthma models, mediated by the chemokines CCL17 and CCL22 [20].
Another important cytokine in the biology of eosinophils, and more broadly in immune regulation, is TGF-β [21, 22]. TGF-β can help control the activation of eosinophils [23–25] and reduce their number in the airways [25, 26] by blocking the pro-survival effects of IL-3, IL-5 and GM-CSF and inducing their apoptosis [19, 27–29]. However, there have been contrasting reports on the effects of TGF-β in the inflamed airways; over-expression of TGF-β in mouse models protected against airway allergy [30], while conversely genetic ablation of TGF-β1 in CD11c+ cells resulted in enhanced allergic eosinophilia [20]. In a different model, however, loss of TGF-β1 expression in epithelial cells muted ILC2 activation and reduced allergic inflammation in mice [31]. Nevertheless, in most settings TGF-β is potently anti-inflammatory [21, 22], particularly through the induction of Foxp3+ T regulatory cells (Tregs), which are essential for induction of tolerance to allergens at mucosal surfaces [32–35] and are known to alleviate experimental airway allergy [36–39].
Type 2-high allergic asthma is increasingly prevalent in the more economically developed countries, particularly in urban communities, where helminth parasite infections are rare. Helminth parasites may broadly suppress type 2 responses to escape the arm of immunity that targets them for expulsion. Interestingly, children with helminth infections show reduced levels of allergic reactivity [40], which is linked to the expansion of Tregs [41] and their ability to suppress airway allergy in mouse models [36, 42]. Furthermore, treatment with anthelmintic drugs to clear parasites results in more rapid acquisition of allergic reactivity [43, 44]. We and others have screened helminth parasites for molecular mediators that might explain reduced allergic status; such products are likely to have been honed by evolution into effective biological inhibitors of the type 2 immune pathway that may offer novel treatments for human asthma [45, 46].
Focussing on Heligmosomoides polygyrus, the intestinal nematode with potent anti-allergic effects [41, 47, 48] we established that H. polygyrus excretory/secretory (HES) products suppress allergic airway inflammation, whether given at sensitisation or at challenge [49]. Two parasite proteins that block sensitisation have been identified as HpARI [50, 51] and HpBARI [52], both of which act on the IL-33 pathway. In searching for further anti-allergic parasite products, we considered a TGF-β mimic, Hp-TGM [53]. Hp-TGM shares no homology to any TGF-β family member, however it binds mammalian TGF-β receptors and induces Foxp3+ Tregs in vitro and in vivo [54–56]. The role of Hp-TGM in Type 2-high asthma remains unexplored.
In this study, we investigated the effects of Hp-TGM in murine models of allergic asthma elicited by A. alternata allergen, HDM or alum-adjuvanted Ovalbumin (OVA) protein. In the simplest model, intranasal administration of Hp-TGM in acute pulmonary inflammation following A. alternata exposure reduced pulmonary eosinophilia 24 h later. Moreover, in models of allergen challenge of sensitized mice, systemic administration of Hp-TGM potently reduced eosinophilic influx in the broncho-alveolar lavage fluid (BALF) and lung, both when given at the time of airway sensitization or at the time of airway challenge. Our data open the door to future research into therapeutic application of helminth-derived molecules to prevent the development or alleviate the symptoms of allergic asthma.
Methods
Reagents
Alternaria alternata extract (Greer Laboratories XPM1D3A25) was resuspended in PBS and concentration assessed by Pierce BCA assay (Thermo Fisher Scientific). HDM extract from Dermatophagoides pteronyssinus (Greer Laboratories XPB70D3A2.5) was resuspended in sterile PBS. Class IV Ovalbumin (Sigma, Gillingham, Dorset, UK) was lipopolysaccharide (LPS) depleted by Triton X-114 phase separation, and the levels of LPS present in HES and depleted OVA were below 0.1 and 0.01 IU LPS per mg protein, respectively by the Limulus Amoebocyte Lysate assay (Lonza, Slough, UK).
Protein expression and purification
Construct encoding Hp-TGM was cloned into the pSec-TAG2A expression vector as previously described, with C-terminal myc and 6-His tags [53]. Purified plasmids were transfected into Expi293F™ cells, and supernatants collected 5 days later. Expi293F™ cells were maintained, and transfections carried out using the Expi293 Expression System according to manufacturer’s instructions (ThermoFisher Scientific). Expressed protein in supernatants were purified over a HisTrap excel column (GE Healthcare) and eluted in a gradient up to 500 mM imidazole. Eluted protein was then dialysed to PBS and filter-sterilized. The protein concentration was determined from A280 nM (Nanodrop, ThermoFisher Scientific), using a calculated extinction coefficient.
Animals
BALB/cAnNCrl mice were purchased from Charles River, UK. RAG1−/− mice and Foxp3-GFP mice were bred at the University of Glasgow. All mice were accommodated and procedures approved by the University of Glasgow Animal Welfare and Ethical Review Board, and performed under UK Home Office licences with institutional oversight performed by qualified veterinarians.
Murine lung and BALF single cell suspension preparation
Lungs were flushed with four washes of 0.5 ml ice-cold PBS to collect BALF cells, followed by lung dissection for single cell preparation. Murine lungs were digested in 2 U/mL of Liberase TL (Roche, Burgess Hill, UK) and 80 U/mL DNase (Life technologies, Paisley, UK) at 37°C with agitation for 35 min. Digested tissue was passed through a 70 μm strainer and red blood cells lysed using Red Blood Cells Lysis Buffer (Sigma). Live cells were counted using a haemocytometer and dead cells excluded using trypan blue.
Alternaria model
Alternaria allergen was used as a model of asthma as previously described [51, 57]. Alternaria allergen (10 μg) and Hp-TGM (5 μg) were administered intranasally to mice under isoflurane anaesthesia and tissues were harvested 24 h after initial Alternaria allergen administration.
House dust mite model
HDM allergen was used as a model of asthma as previously described [58]. Mice were anaesthetized with iso-flurane, sensitized intranasally (i.n.) with 3 μg HDM in 50 μl PBS. After 7 days, mice were challenged with 10 μg HDM on five consecutive days under anaesthesia. Three days after the last challenge, mice were sacrificed, and tissues were harvested. Hp-TGM (1 μg) was administered i.n. or intraperitoneally (i.p.), as indicated.
OVA/alum model
The OVA/Alum was used as a model of asthma as previously described [59]. Mice were anaesthetized with isoflurane and intraperitoneally administered 200 μl of PBS-Imject Alum containing 20 μg Ovalbumin (OVA) (final ratio of 1:1 Imject Alum to OVA). Day 7–9 post sensitisation, mice were briefly anaesthetised with isoflurane and intranasally (i.n.) administered 50 μl PBS containing 20 μg Ovalbumin. Twenty-four hours after the last challenge, mice were sacrificed and tissues were harvested. Hp-TGM (1 μg) was administered intraperitoneally (i.p.), as indicated.
Flow cytometry staining
For surface staining, single cell suspensions from lung or BALF were washed in PBS and stained with Fixable Blue Live/Dead stain (Thermo Fisher). Cells were then blocked with anti-mouse CD16/32 antibody (Biolegend) and surface stained. BALF and lung cells were labelled with CD3 (FITC, clone 145-2C11, Biolegend), Siglec-F (PE, clone REA798, Miltenyi), Ly6G (PerCP-Cy5.5, clone 1A8, Biolegend), CD11c (AF647, clone N418, Biolegend), CD11b (Pacific Blue, clone M1/70, Biolegend), CD45 (AF700, clone 30-F11 Invitrogen), CD4 (PE-Dazzle, clone RM4.5, Biolegend), Lineage marker cocktail composed of FITC-labelled CD3 (clone 145-2C11, Biolegend), CD5 (clone 53–7.3, Biolegend), CD11b (M1/70.15, Invitrogen), CD19 (clone 6D5, Biolegend), CD49b (clone DX5, eBioscience) and GR1 (clone RB6-8C5, Biolegend), ICOS (PerCP Cy5.5, clone C398.4A, Biolegend), ST2 (APC, clone DIH9-2, Biolegend), CD25 (BV650, clone PC61, Biolegend), CCR3 (PerCP/Cyanine5.5, clone J073E5, Biolegend), and CD69 (PE, clone H1.2F3, Biolegend).
For intracellular cytokine staining, single lung cell suspensions were stimulated with PMA (500 ng/ml), ionomycin (1 μg/ml) and brefeldin A (10 μg/ml) for 4 h at 37° C, 5% CO2. Stimulated cells were surface stained, and permeabilised with IC permeabilization buffer following manufacturer instructions (eBioscience). For intracellular cytokine staining, cells were stained for IL-5 (PE, clone TRFK5, Biolegend) and IL-13 (PECy7, clone eBio13A, Biolegend). For Foxp3 (eF450, clone FJK-16s, eBioscience) staining, cells were fixed and permeabilised with Foxp3/transcription factor staining buffer following manufacturer instructions (eBioscience).
RNA extraction, reverse transcription and qPCR
Lung tissue was placed in RNALater Stabilizing Solution (Thermo Fisher Scientific) and stored at −20°C. Lung tissues were then transferred from RNALater to TRIzol (Thermo Fisher Scientific) and homogenized using 3 mm stainless steel beads (Qiagen) in a TissueLyser II (Qiagen) at 25 Hz for 2 min.
Complementary DNA was made using qScript® cDNA Synthesis Kit (Quantabio). Primers were pur-chased from Life Technologies and amplification reaction was carried out using PerfeCTa® SYBR® Green Super-Mix, Low ROX (Quantabio) in a 13 μl volume made up of 0.25 μl of nuclease free water, 0.25 μl forward primer, 0.25 μl reverse primer, 6.25 μl of 2x concentrated reaction mix and 6 μl DNA template (10 ng). Amplification was carried out using QuantStudio and 384-well plate (Applied Biosystems). PCR data were analysed using QuantStudio Real-Time PCR software v1.1 and the 2-ΔΔCT method. In brief, relative gene expression between different samples was calculated using the threshold cycles (CTs) generated by QuantStudio 384-well plate machine and software. ΔCT for each sample was calculated subtracting CT of the housekeeping gene (HPRT-1) from the CT of the gene of interest. To obtain the relative gene expression between control group and treatment, ΔΔCTs were then obtained subtracting the average of the control group ΔCTs (e.g., PBS) from the ΔCT of the sample. Subsequently, 2-ΔΔCT was calculated and plotted in a graph.
Cytokine measurement
Cytokine measurements by Luminex assays (ProcartaPlex, ThermoFisher Scientific) and ELISAs for IL-4, IL-5, IL-13 (Ready-SET-go, ThermoFisher Scientific) and CCL-11 (Eotaxin-1; DuoSet, Bio-Techne) were carried out to manufacturer’s instructions.
Statistical analysis
All data were analysed using Prism (Graphpad Software Inc.), one-way ANOVA with Tukey’s multiple comparisons post-test was used to compare multiple independent groups. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns = not significant (p > 0.05). Where significant effects were observed for physiological outcomes (e.g., eosinophilia) similar group sizes were deemed sufficient to test individual parameters, such as cytokines.
Results
Hp-TGM inhibits innate allergic airway responses
We first evaluated the ability of Hp-TGM to influence the early innate pro-allergic response to the fungal allergen A. alternata 24 h after intranasal administration. This model was previously shown to be highly sensitive to inhibition by total H. polygyrus HES, and key IL-33-pathway inhibitors in HES, HpARI and HpBARI [51, 52, 57]. In this system, we tested the effect of co-administration of Hp-TGM and A. alternata extract by the intranasal route (Figure 1a). We analysed differential cell responses by flow cytometry using the gating strategies shown for BALF (Supplementary Figure 1) and lung (Supplementary Figure 2) cells. BALF contents broadly represent responses of airway epithelial cells and resident or recruited immune cells to allergen exposure, while analyses of whole lung homogenates also reflect changes in the lung parenchyma.
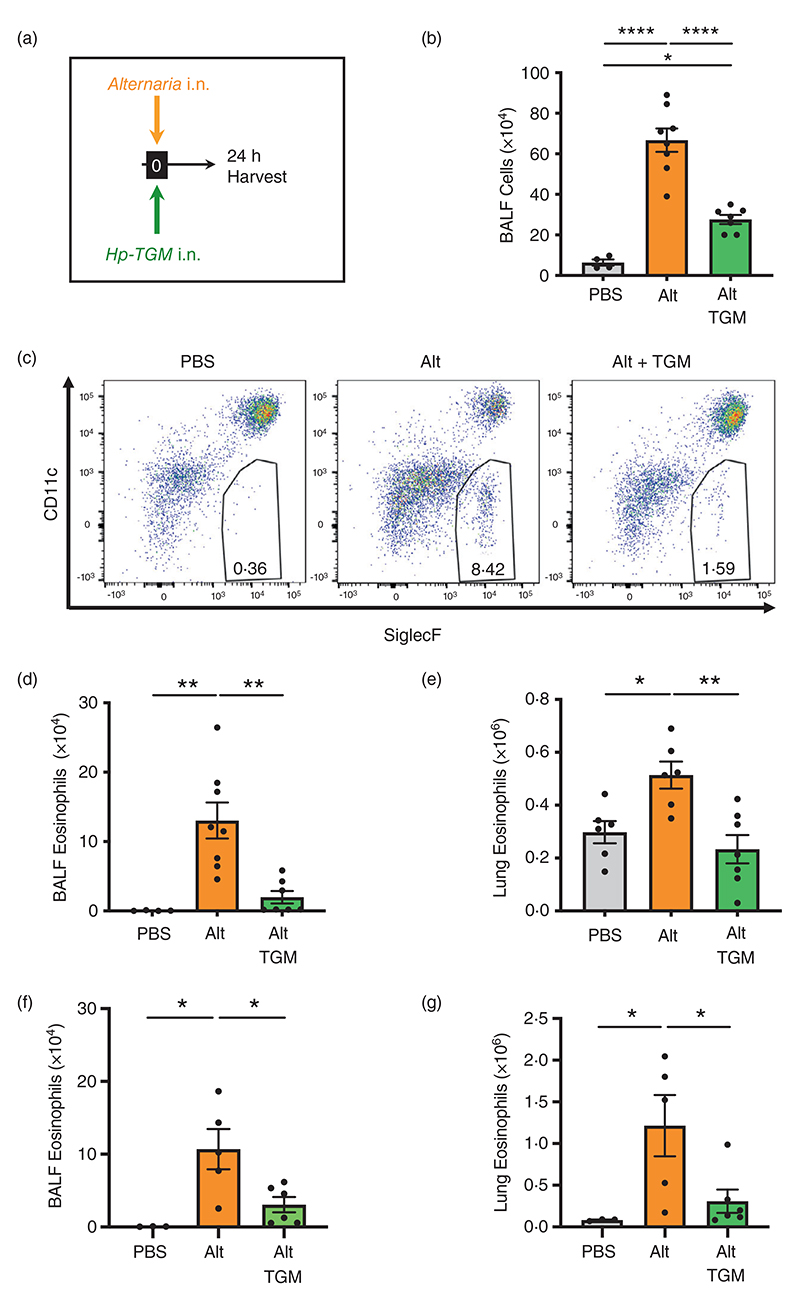
Figure 1.
Hp-TGM reduces airway eosinophilia in response to Alternaria allergen. Alternaria allergen (10 μg) mixed with or without Hp-TGM (5 μg) was intranasally administered and mice culled 24 h later for recovery of bronchoalveolar lavage fluids (BALFs) and lung tissues. (a) Schematic representation of the experimental protocol. (b) Total cell numbers in BALF from control mice and those receiving Alternaria allergen (Alt) or Alternaria with Hp-TGM (Alt-TGM). (c) Representative flow cytometry plots of SiglecF vs. CD11c expression on CD45+ BALF cells from individual mice from the three experimental groups; percentage of eosinophils shown in inset boxes for each plot. (d) Numbers of eosinophils (CD45+CD11b+CD11c−Ly6C−Ly6G−SiglecF+) in BALF determined by flow cytometry. (d) Numbers of eosinophils (CD45+CD11b+CD11c−Ly6C−Ly6G−SiglecF+) in lung homogenates determined by flow cvtometrv. (f and g) Numbers of eosinophils (CD45+CD11b+CD11c−Ly6C−Ly6G−SiglecF+) in BALF (f) and lung homogenates (g) of RAG-1 deficient mice determined by flow cytometry. Results are pooled from two independent experiments each with 2–4 mice per group. All data were analvsed using Prism (Graphpad Software Inc.), one-wav ANOVA with Tukev’s multiple comparisons post-test was used to compare multiple independent groups. Mean values and SEM are indicated. ****p < 0.0001; **p < 0.01; *p < 0.05; non-significant differences not shown.
In the BALF, A. alternata induced strong airway inflammation and eosinophilia which was highly suppressed by co-administration of Hp-TGM, as measured by total cellular infiltrate into the BALF (Figure 1b) and total eosinophils within the infiltrating population (Figure 1c,d). In the lung, eosinophilia developed within 24 h of A. alternata treatment, but this was abolished by co-administration of Hp-TGM (Figure 1e). Hp-TGM was also effective in abrogating A. alternata-induced eosinophilia in RAG-1-deficient mice (Figure 1f,g), confirming that innate responses were suppressed directly without involvement of adaptive lymphocytes. Furthermore, Hp-TGM inhibition of A. alternata-induced pulmonary eosinophilia was observed to act in a dose-dependent manner with diminution evident even when 5 ng of the parasite mediator was administered (Supplementary Figure 3A).
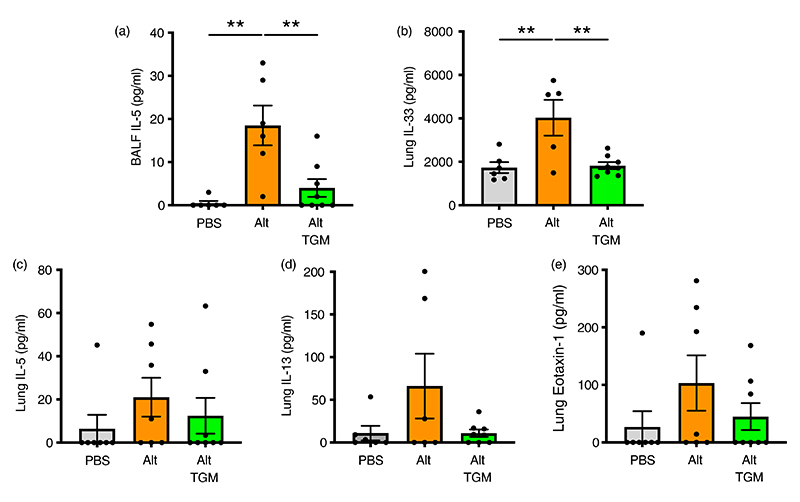
We then measured type 2 cytokines in BALF and lung homogenate; in BALF, IL-5 levels induced by A. alternata were substantially reduced when the allergen was coadministered with Hp-TGM (Figure 2a). BALF IL-13 levels were marginally above the limit of detection in some, but not all animals (data not shown), and Hp-TGM exerted no significant effect. In the lung, IL-33 in lung homogenates was fully suppressed to baseline levels in the presence of Hp-TGM (Figure 2b), while IL-5, IL-13 and eotaxin-1 were reduced to different degrees, albeit falling short of significance (Figure 2c–e). We also analysed type 2 innate lymphoid cells (ILC2s), which are known to respond to IL-33 and contribute IL-13 and IL-5 to the early allergic response, but noted no overt effect of Hp-TGM on their expression by flow cytometry (Supplementary Figure 3B,C).
Figure 2.
Cytokine responses in Alternaria-challenged airways. Mice were treated with Alternaria allergen with or without Hp-TGM as described for Figure 1 and samples recovered at 24 h. (a) IL-5 cytokine measured in cell-free BALF supernatants. (b–e) IL-33 (b), IL-5 (c), IL-13 (d), eotaxin-1 (e) cytokines measured in lung homogenates. Results are pooled from two independent experiments each with 3–4 mice per group. All data were analysed using Prism (Graphpad Software Inc.), one-way ANOVA with Tukey’s multiple comparisons post-test was used to compare multiple independent groups. Mean values and SEM are indicated. **p < 0.01; *p < 0.05; non-significant differences not shown.
Hp-TGM is protective in a T cell-dependent model of airway allergy
Because allergy is primarily mediated by a type 2 adaptive immune response, we then tested Hp-TGM in a T cell-dependent model of airway sensitization and challenge, using HDM extract [60], with intraperitoneal administration of Hp-TGM during allergen sensitization (day 0) or at each day of challenge (day 7–11) (Figure 3a). In this context, a single exposure to Hp-TGM on the day of sensitisation was sufficient to strongly inhibit total cellular influx into the BALF when assessed 4 days after the final challenge dose (Figure 3b). A substantial reduction in eosinophilia was seen although not reaching statistical significance (Figure 3c). In the lung, while total cellularity did not differ greatly (Figure 3d), eosinophilia (Figure 3e,f) was strongly down-modulated in animals receiving Hp-TGM at the time of sensitization.
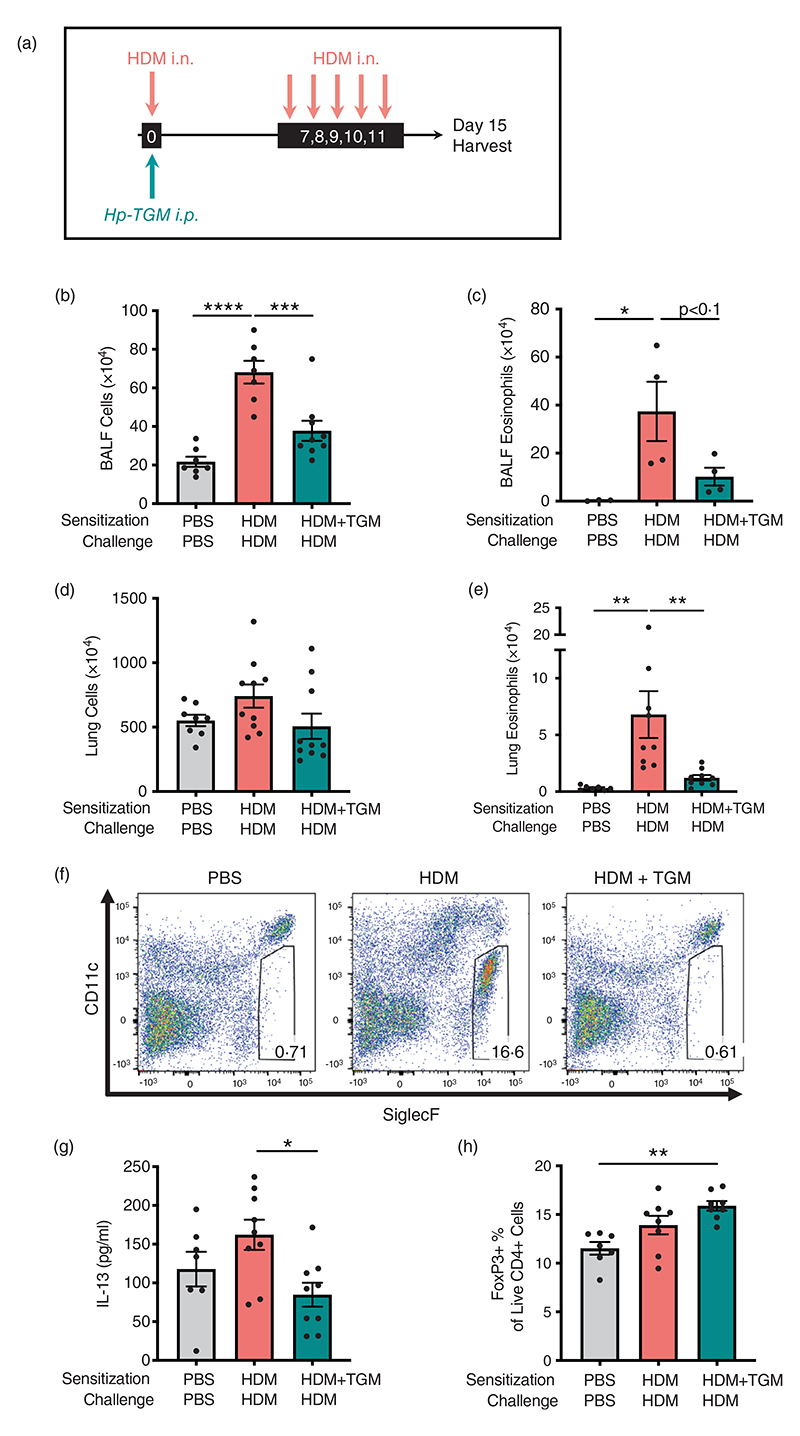
Figure 3.
Hp-TGM reduces allergic sensitization to house dust mite. Mice were sensitized with house dust mite allergen (3 μg) intranasally (i.n.) and Hp-TGM (1 μg) was administered intraperitoneally (i.p.) at day 0. Allergic responses to HDM were restimulated daily from day 7 to day 11 (10 μg) and mice culled at day 15. (a) Schematic representation of the experimental protocol. (b and c) Total cell (b) and eosinophil (c) cell numbers were enumerated in the BALF. (d) Total cell numbers enumerated in lung homogenates. (e and f) Eosinophil cell numbers enumerated in lung homogenates (e) and representative flow cytometry plot of SiglecF vs. CD11c expression on lung cells gated on live CD45+ cells. Percentage of eosinophils shown in inset boxes for each plot (f). (g) IL-13 measured by ELISA in lung homogenates. (h) Foxp3 expression on lung cells gated on live CD4+ cells from control mice and those receiving HDM with or without Hp-TGM. Data are from ≥2 independent experiments (n = 6–9), except panel (c) which is from one representative experiment (n = 4–5). All data were analysed using Prism (Graphpad Software Inc.), one-way ANOVA with Tukey’s multiple comparisons post-test was used to compare multiple independent groups. Mean values and SEM are indicated. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; nonsignificant differences not shown.
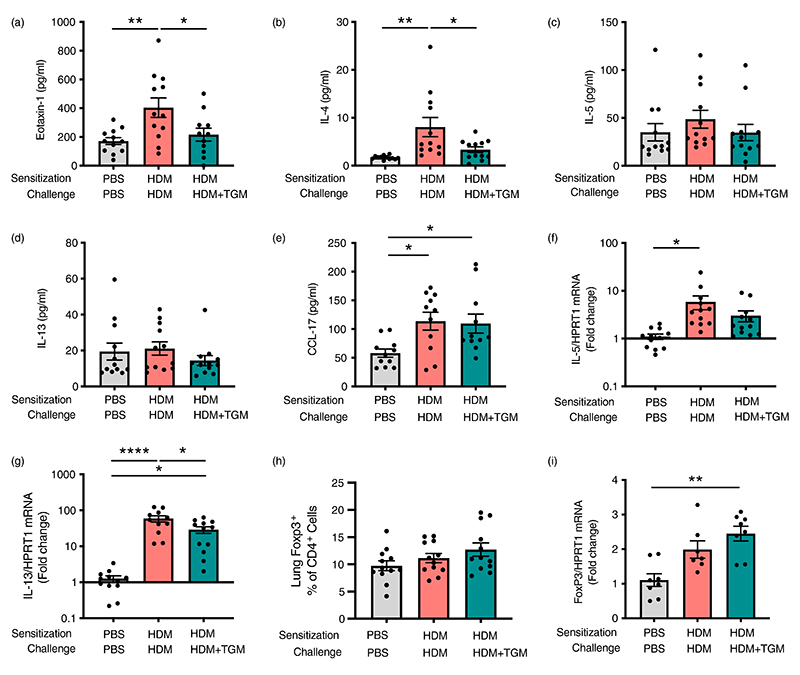
Levels of type 2 cytokines in lung homogenates on day 15 were then analysed. Hp-TGM administration strongly inhibited IL-13 responses in animals receiving HDM (Figure 3g), while other cytokines (IL-4, IL-5 and eotaxin-1) were not significantly induced by HDM in these experiments and no significant differences in these cytokines were observed with Hp-TGM administration (data not shown). As Hp-TGM is a potent inducer of CD25+Foxp3+ regulatory T cells, we also evaluated their levels in the lungs of mice in these experiments. As shown in Figure 3h, the proportion of Foxp3+ Tregs differed only slightly between the groups, although compared to naïve controls there was a significant increase in mice receiving Hp-TGM on the day of allergic sensitization with HDM.
Hp-TGM sharply reduces allergic eosinophilia in response to allergen challenge
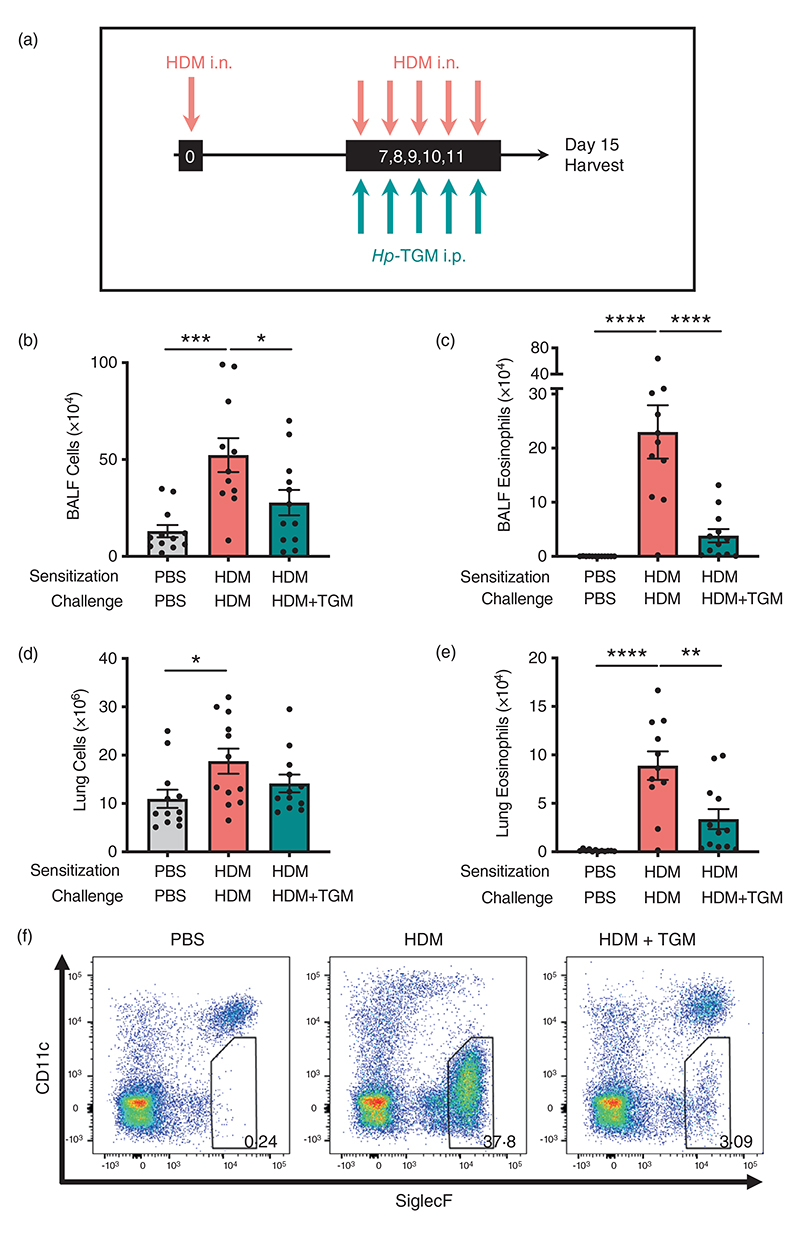
To further investigate the potential of Hp-TGM as a therapeutic option for airway allergy, we next tested its ability to dampen allergic inflammation in previously sensitized animals. Mice were sensitized with HDM at day 0 and challenged with the same allergen for five consecutive days from day 7 to day 11 after sensitization. On the same day of each HDM challenge, mice received either Hp-TGM or PBS i.p. (Figure 4a). Four days following the final challenge, total cell influx, lymphocyte infiltration and eosinophilia were measured in the BALF and lung. In mice that received Hp-TGM, total cell numbers were sharply reduced in the BALF (Figure 4b) with a highly suppressive effect on eosinophilia (Figure 4c). In the lung, although the total cellularity was not greatly reduced (Figure 4d), a strong inhibition of eosinophilia was again evident (Figure 4e,f).
Figure 4.
Hp-TGM administrated at challenge reduces allergic responses to house dust mite. Mice were sensitized with house dust mite allergen (3 μg) intranasally at day 0. Allergic responses to HDM were recalled daily from day 7 to day 11 (10 μg) and Hp-TGM (1 μg) was administered intraperitoneally every day of challenge. Mice were then culled at day 15. (a) Schematic representation of the experimental protocol. (b and c) Total cell (b) and eosinophil (c) numbers were enumerated in BALF. (d) Total cell numbers enumerated in lung homogenates. (e and f) Eosinophil cell numbers enumerated in lung homogenates (e) and representative flow cytometry plot of SiglecF versus CD11c expression on lung cells gated on live CD45+ cells. Percentage of eosinophils shown in inset boxes for each plot (f). Data are from ≥2 independent experiments (n = 7-9). All data were analysed using Prism (Graphpad Software Inc.), one-way ANOVA with Tukey’s multiple comparisons post-test was used to compare multiple independent groups. Mean values and SEM are indicated. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; nonsignificant differences not shown.
Analysis of BAL and lung neutrophil (Supplementary Figure 4A,C, respectively) and alveolar macrophage (Supplementary Figure 4B,D, respectively) populations showed similar responses in mice challenged with HDM, irrespective of the presence or absence of Hp-TGM administration. Similarly, key cell types in the pulmonary tissues found only modest differences in Th2 (Supplementary Figure 5I) and ILC2 (Supplementary Figure 5J) cell numbers, neither of which reached statistical significance.
Suppression of eosinophilia by systemic Hp-TGM is associated with significant reduction of eotaxin-1 and IL-4 responses, rather than induction of regulatory responses
We then examined if the effect of Hp-TGM during allergen challenge was accompanied by a modulation of type 2 cytokines in the lung tissues. Most notably, eotaxin-1 (Figure 5a) and IL-4 (Figure 5b) were significantly attenuated in Hp-TGM-treated mice, although at this time point IL-5 (Figure 5c) and IL-13 (Figure 5d) were not different between groups. An increase in CCL17 chemokine levels in HDM-allergic mice was similar irrespective of Hp-TGM administration (Figure 5e). We also examined mRNA transcript levels for these and other cytokines, finding no effect on eotaxin-1, IL-4 (Supplementary Figure 5A,B), or IL-5 (Figure 5f), but a significant decrease in IL-13 transcripts following Hp-TGM treatment (Figure 5g). Of note, no differences in BALF cytokines were observed (data not shown). We also measured GM-CSF and IL-3 protein levels in lung homogenates, however, no effect of Hp-TGM were observed on these molecules (Supplementary Figure 5C,D).
Figure 5.
Inhibition of eosinophilia by Hp-TGM is associated with lower IL-4 and eotaxin-1 responses. Using the same protocol as previously described (Figure 4a), type 2 cytokine and regulatory gene responses were assessed by Luminex and/or qPCR. (a–e) Lung homogenate levels of eotaxin-1, IL-4, IL-5, IL-13 and CCL17 were measured. (f and g) Expression of Il5 and Il13 transcripts. (h) Percentage of Foxp3+ within all CD4+ T cells. (i) Expression of Foxp3 transcripts in lung homogenates. Data are from ≥2 independent experiments, Data shown are pooled from two experiments (n = 7–9). All data were analysed using Prism (Graphpad Software Inc.), one-way ANOVA with Tukey’s multiple comparisons post-test was used to compare multiple independent groups. Mean values and SEM are indicated. ****p < 0.0001; **p < 0.01; *p < 0.05; non-significant differences not shown.
In addition, we investigated if Hp-TGM effect on allergic eosinophilia involved induction of Tregs and their regulatory cytokines, since we and others have previously shown their importance in the suppression of airway allergy [36, 61, 62]. However, the percentage of Tregs in CD4+ T cells (Figure 5h) and levels of Foxp3 mRNA (Figure 5i) were only marginally increased following parenteral Hp-TGM and did not reach significance. Similarly, IL-10 protein and mRNA transcript levels (Supplementary Figure 5E,F), and TGFβ mRNA levels (Supplementary Figure 5G) were unchanged by Hp-TGM administration. Interestingly, HDM treatment resulted in a significant reduction in IL-4Rα transcripts compared to both control and Hp-TGM-treated mice (Supplementary Figure 5H), which may represent receptor downregulation following a higher level of activation.
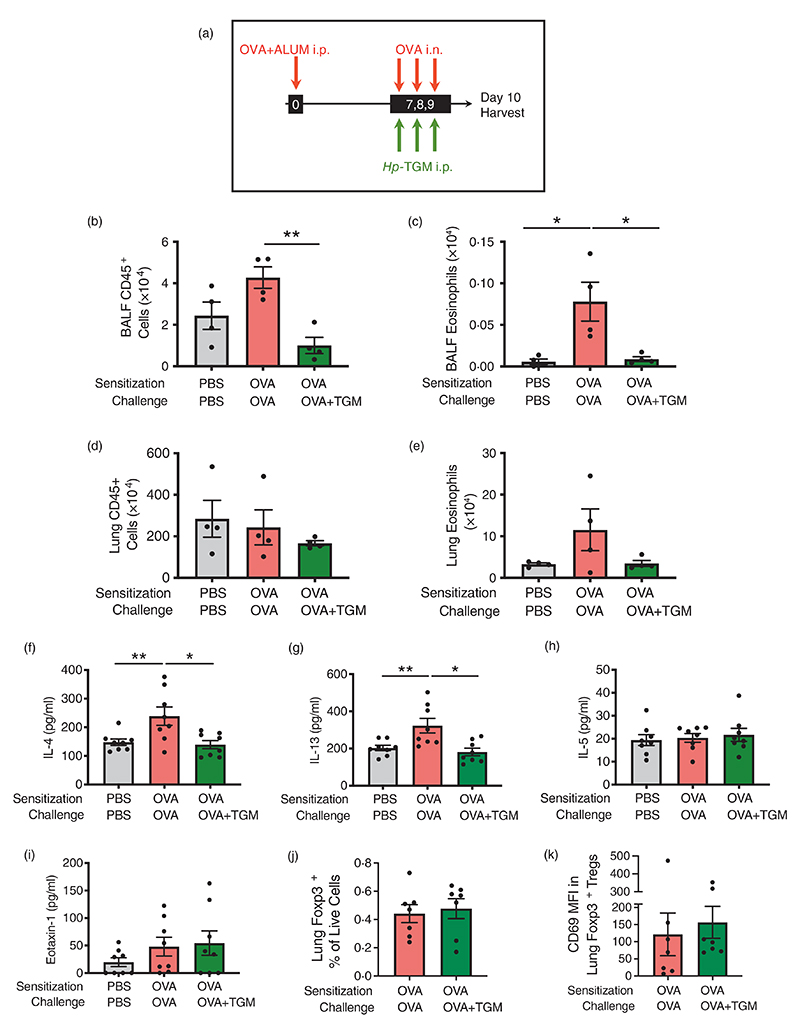
Hp-TGM also reduces airway eosinophilia in the ovalbumin allergy model
Although ovalbumin-induced models are less clinically-relevant than airway allergen models, since sensitization is achieved by intraperitoneal administration of OVA protein emulsified in alum, they are characterized by a Th2 response with IL-4, IL-5, and IL-13 production, and are independent of epithelial-derived alarmin cytokines, such as IL-33 [1]. To check if parenteral Hp-TGM could also control eosinophilia in this allergy model, we administered a pre-mixed OVA/Alum solution intraperitoneally to mice at day 0, and recalled responses to OVA by administering it intranasally from day 7 to day 9 following injections with Hp-TGM 3 h earlier on the same day. On day 10, mice were culled, and airway tissues harvested (Figure 6a). In this setting, Hp-TGM significantly decreased CD45+ cell influx in the BALF (Figure 6b) and almost completely abolished eosinophilia (Figure 6c). In the lung, cellularity was little changed (Figure 6d) and while there was a strong trend for reduction of eosinophilia in the presence of Hp-TGM, this did not reach significance (Figure 6e). In addition, IL-4 and IL-13 were significantly reduced in lung homogenates of mice receiving Hp-TGM, while IL-5 and eotaxin-1 responses were little affected (Figure 6f–i). No significant differences in CCR3 (eotaxin-1 receptor) expression were seen between the different groups (Supplementary Figure 6A,B). Furthermore, no significant changes were seen in the profile of lung Foxp3+ Tregs as a results of Hp-TGM treatment, either in terms of frequency (Figure 6j) or induction of the activation marker CD69 within the Treg population (Figure 6k). Taken together, these data support the hypothesis of a regulation of T cell-mediated allergic airway eosinophilia by Hp-TGM via modulation of IL-4 and IL-13 responses.
Figure 6.
Hp-TGM blocks allergic eosinophilia during an OVA challenge. Mice were sensitized with Ovalbumin (OVA, 20 μg) intraperitoneally at day 0. OVA was then intranasally administered daily from day 7 to day 9 (20 μg) and Hp-TGM (1 μg) was administered intraperitoneally every day of challenge. Mice were then culled at day 10. (a) Schematic representation of the experimental protocol. (b–e) CD45+ cell and eosinophil cell numbers were enumerated in BALF (b and c) and lung (d and e). (f–i) Type 2 cytokines were measured in lung homogenates, showing IL-4, IL-13, IL-5 and eotaxin-1. (j) Frequency of Foxp3+ Tregs in lung homogenates of mice sensitized and challenged with OVA, in the absence or presence of Hp-TGM administration. (k) Expression of CD69 within the Foxp3+ Treg population in lung homogenates of mice sensitized and challenged with OVA, in the absence or presence of Hp-TGM administration. Data are from ≥2 independent experiments. Data shown are pooled from two experiments (n = 7–9). All data were analysed using Prism (Graphpad Software Inc.), one-way ANOVA with Tukey’s multiple comparisons post-test was used to compare multiple independent groups. Mean values and SEM are indicated. **p < 0.01; *p < 0.05; non-significant differences not shown.
Discussion
Helminth parasites are frequently associated with protection from allergic reactivity [40, 42, 63, 64]. Although deliberate infection with helminths has been trialled for the alleviation of inflammatory symptoms [65–67], future helminth-derived therapies will require defined products which can be individually evaluated as potential pharmacological agents [45]. In this report, we show that the helminth-derived TGF-β mimic, Hp-TGM, potently suppressed allergic eosinophilia in three different experimental models of airway inflammation, in each case revealing specific cellular and cytokine modulation occurring in vivo.
In the first model, we appraised short-term innate responses 24 h following A. alternata exposure. Eosinophilia was sharply reduced by Hp-TGM co-administration, indicating direct effect(s) on innate cell populations, together with overall diminution of type 2 cytokines, in particular BALF IL-5 and lung IL-33, the latter known to be an essential driver of immune responses in this model [68–70]. In mice, IL-33 is predominantly released by stromal cells in the lung tissue, including epithelial cells, fibroblasts, and endothelial cells [71, 72], while in mast cells and dendritic cells IL-33 can be induced under inflammatory conditions [73, 74]. Ongoing studies aim to determine which of these populations may be down-regulated by Hp-TGM in this mode. Lung ILC2s release IL-5 and IL-13 in response to IL-33 [75, 76], but were not significantly affected by Hp-TGM. One cell type that also produces IL-5 and IL-13 in response to IL-33 is the mast cell [77], and as TGF-β has been shown to suppress IL-33–induced mast cell function in the context of asthma [78], this population is a prime candidate for further investigation.
We next tested Hp-TGM effects in airway allergy models where type 2 adaptive immune responses are involved and found significant protection when Hp-TGM was administered systemically. Suppression of eosinophilia was marked, both when Hp-TGM was given at the time of sensitization, or during repeated challenge, and again we found significant inhibition of eotaxin-1, IL-4 and/or IL-13 responses, depending on the model and the time-points examined. Similar effects on eosinophils were observed with HDM, or the model antigen Ovalbumin (OVA). However, in none of the protocols did Hp-TGM particularly affect Th2 and ILC2 cell numbers, and its effects on Foxp3+ regulatory T (Treg) cells were modest at best. This is in accordance with previous data from our group showing that administration of HES in allergic asthma models suppressed airway eosinophilia without inducing Treg responses [49].
Eotaxin-1 and IL-4 are important cytokine in eosinophil migration to inflamed sites. Indeed, IL-4 has been shown to induce the expression of adhesion molecules ICAM-1 (intercellular cell adhesion molecule-1) and VCAM-1 (vascular cell adhesion molecule-1) that allow eosinophils to exit the inflamed airways [79, 80], as well as to promote eosinophil chemotaxis by inducing eotaxin-1 in epithelial cells. We previously reported reduction of eotaxin-1 in allergy models in the presence of HES [49], which we now suggest may be mediated by Hp-TGM.
The source of IL-4 cytokine targeted by Hp-TGM in our models remain unknown, however, T follicular helper cells (Tfh) may well be the primary target of Hp-TGM since they are the main producer of IL-4 in allergic asthma [70, 81, 82]. We have yet to investigate the possible involvement of other immune cells known to produce IL-4 in the context of asthma, such as basophils [83–87] or activated ILC2s [70]. Notably, we found that Hp-TGM not only reduced lung IL-4 cytokine levels, but also downregulated IL-4Rα gene expression to baseline. Within the T cell population, which drives the response to HDM, IL-4Rα downregulation is induced by TCR engagement which renders the cell unresponsive to IL-4 [88]. Our data therefore suggest that Hp-TGM may limit Th cell antigenic stimulation, as part of a wider picture in which administration of HES in allergy models reduces Th2 responses without skewing responses towards Th1 cells [49].
Finally, in all models tested, Hp-TGM could have affected the eosinophil population directly. Indeed, TGF-β has been shown to downregulate eosinophil responses by various means, for example, by inducing their apoptosis [22, 27, 28], limiting their differentiation from bone marrow precursors, limiting their proliferation by antagonizing the effect of IL-3, IL-5 and GM-CSF [19, 22, 89, 90], by limiting their activation via downregulation of the eosinophils activation marker HLA-DR [91], or by retaining them in noninflamed tissues by enhancing expression of the chemokine receptor CXCR4 [22, 92]. Although we did not directly compare the effect of Hp-TGM to TGF-β, we have previously compared the effect of TGF-β and HES in similar asthma models [49, 93]. While intravenous recombinant TGF-β was shown to suppress development of Th2-mediated pathology, including airway eosinophilia the effect was mediated by the induction of Tregs [93]. Intranasal TGF-β did not show any suppression of eosinophilic BALF nor modulated Th2 cytokines [49]. In our models, systemic Hp-TGM did not significantly expand Treg frequencies nor modulated IL-3, IL-5 or GM-CSF responses. Thus, it is possible that Hp-TGM could have a different mode of action than TGF-β to control airway eosinophilia.
In summary, we present data showing that Hp-TGM administration can recapitulate generalized anti-allergic effects that were previously found for the complex mixture of H. polygyrus ES products (HES) [49, 93]. Airway epithelial cells are likely to be central in Hp-TGM effect on airway eosinophilia since Hp-TGM reduced the levels of IL-33 cytokine in the Alternaria model and eotaxin-1 levels in the HDM model, both of which are primarily epithelial-derived and underpin eosinophil accumulation in the allergic lung [70]. To our knowledge this is the first time that a parasite-derived protein has been described to reduce airway allergic eosinophilia on allergen rechallenge by acting on epithelial-derived cytokines and IL-4 responses rather than on the classical IL-5 cytokine response. We believe that Hp-TGM may contain possible preventive or therapeutic potential for human allergic airway disease, as targeting eosinophil infiltration in the lung has already proven to be successful in controlling type 2-high asthma in clinical trials [3, 4, 8, 9, 11–13]. Future work will explore this possibility in depth.
Supplementary Material
Acknowledgements
Caroline Chauché, Orhan Rasid, Anne-Marie Donachie, Danielle J. Smyth, Henry J. McSorley and Rick M. Maizels thank the Lung Foundation Netherlands (LongFonds) for support through the Accelerate consortium ‘A World Without Asthma’; Caroline Chauché was also supported by a veterinary postdoctoral research fellowship funded by the Horserace Betting Levy Board (VET/2020-1 EPDF 7), Caitlin M. McManus by a University of Glasgow PhD Studentship, and Stephan Löser, Josh Richards, Tiffany Campion and Danielle J. Smyth were supported by the Wellcome Trust through an Investigator Award to RMM (Ref.: 219530), and the Wellcome Trust core-funded Wellcome Centre for Integrative Parasitology (Ref.: 104111). We gratefully acknowledge assistance and expertise from the Flow Core Facility and the Wolfson Research Facility at the University of Glasgow.
Funding information
Horserace Betting Levy Board, Grant/Award Number: VET/2020-1 EPDF7; Lung Foundation Netherlands; Wellcome Trust, Grant/Award Numbers: 104111, 219530; University of Glasgow PhD Studentship
Footnotes
Conflict of Interest
The authors declare that they have no competing financial interests.
Supporting Information
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184:1469–85. doi: 10.1016/j.cell.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Smits HH, van der Vlugt LE, von Mutius E, Hiemstra PS. Childhood allergies and asthma: new insights on environmental exposures and local immunity at the lung barrier. Curr Opin Immunol. 2016;42:41–7. doi: 10.1016/j.coi.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 5.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12:117–29. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graff S, Bricmont N, Moermans C, Henket M, Paulus V, Guissard F, et al. Clinical and biological factors associated with irreversible airway obstruction in adult asthma. Respir Med. 2020;175:106202. doi: 10.1016/j.rmed.2020.106202. [DOI] [PubMed] [Google Scholar]
- 7.McBrien CN, Menzies-Gow A. The biology of eosinophils and their role in asthma. Front Med (Lausanne) 2017;4:93. doi: 10.3389/fmed.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg ME. Humanized anti-IL-5 antibody therapy. Cell. 2016;165:509. doi: 10.1016/j.cell.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cushen B, Menzies-Gow A. Benralizumab: an updated treatment of eosinophilic asthma. Expert Rev Respir Med. 2020;14:435–44. doi: 10.1080/17476348.2020.1739526. [DOI] [PubMed] [Google Scholar]
- 11.de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1:00024-2015. doi: 10.1183/23120541.00024-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landolina NA, Levi-Schaffer F. Eosinophils as a pharmacological target for the treatment of allergic diseases. Curr Opin Pharmacol. 2014;17:71–80. doi: 10.1016/j.coph.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, et al. Th2 cells in health and disease. Annu Rev Immunol. 2017;35:53–84. doi: 10.1146/annurev-immunol-051116-052350. [DOI] [PubMed] [Google Scholar]
- 14.Pavord ID, Menzies-Gow A, Buhl R, Chanez P, Dransfield M, Lugogo N, et al. Clinical development of mepolizumab for the treatment of severe eosinophilic asthma: on the path to personalized medicine. J Allergy Clin Immunol Pract. 2021;9:1121–1132.:e1127. doi: 10.1016/j.jaip.2020.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–86. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- 16.Godar M, Deswarte K, Vergote K, Saunders M, de Haard H, Hammad H, et al. A bispecific antibody strategy to target multiple type 2 cytokines in asthma. J Allergy Clin Immunol. 2018;142:1185–1193.:e1184. doi: 10.1016/j.jaci.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42:294–308. doi: 10.1016/j.immuni.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor β abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994;179:1041–5. doi: 10.1084/jem.179.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi S, Zhai J, Niu R, Zhu G, Wang M, Liu J, et al. Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge. Nat Commun. 2018;9:3879. doi: 10.1038/s41467-018-06316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 22.Kelly A, Houston SA, Sherwood E, Casulli J, Travis MA. Regulation of innate and adaptive immunity by TGFβ. Adv Immunol. 2017;134:137–233. doi: 10.1016/bs.ai.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Kanzaki M, Shibagaki N, Hatsushika K, Mitsui H, Inozume T, Okamoto A, et al. Human eosinophils have an intact Smad signaling pathway leading to a major transforming growth factor-β target gene expression. Int Arch Allergy Immunol. 2007;142:309–17. doi: 10.1159/000097500. [DOI] [PubMed] [Google Scholar]
- 24.Myrtek D, Knoll M, Matthiesen T, Krause S, Lohrmann J, Schillinger D, et al. Expression of interleukin-13 receptor α1-subunit on peripheral blood eosinophils is regulated by cytokines. Immunology. 2004;112:597–604. doi: 10.1046/j.1365-2567.2004.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore B, Murphy RF, Agrawal DK. Interaction of TGF-β with immune cells in airway disease. Curr Mol Med. 2008;8:427–36. doi: 10.2174/156652408785160943. [DOI] [PubMed] [Google Scholar]
- 26.Fu CL, Ye YL, Lee YL, Chiang BL. Effects of overexpression of IL-10, IL-12, TGF-β and IL-4 on allergen induced change in bronchial responsiveness. Respir Res. 2006;7:72. doi: 10.1186/1465-9921-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Q, Shen ZJ, Oh J, Chu H, Malter JS. Transforming growth factor-β1 antagonizes Interleukin-5 pro-survival signaling by activating calpain-1 in primary human eosinophils. J Clin Cell Immunol. 2011;S1:003. doi: 10.4172/2155-9899.S1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen ZJ, Hu J, Esnault S, Dozmorov I, Malter JS. RNA Seq profiling reveals a novel expression pattern of TGF-β target genes in human blood eosinophils. Immunol Lett. 2015;167:1–10. doi: 10.1016/j.imlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goplen N, Gorska MM, Stafford SJ, Rozario S, Guo L, Liang Q, et al. A phosphosite screen identifies autocrine TGF-β-driven activation of protein kinase R as a survival-limiting factor for eosinophils. J Immunol. 2008;180:4256–64. doi: 10.4049/jimmunol.180.6.4256. [DOI] [PubMed] [Google Scholar]
- 30.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, et al. CD4+ T helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest. 2000;105:61–70. doi: 10.1172/JCI7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denney L, Byrne AJ, Shea TJ, Buckley JS, Pease JE, Herledan GMF, et al. Pulmonary epithelial cell-derived cytokine TGF-β1 is a critical cofactor for enhanced innate lymphoid cell function. Immunity. 2015;43:945–58. doi: 10.1016/j.immuni.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and nonself. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 33.Georgiev P, Charbonnier LM, Chatila TA. Regulatory T cells: the many faces of Foxp3. J Clin Immunol. 2019;39:623–40. doi: 10.1007/s10875-019-00684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, Openshaw PJ. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol. 2013;87:10946–54. doi: 10.1128/JVI.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whibley N, Tucci A, Powrie F. Regulatory T cell adaptation in the intestine and skin. Nat Immunol. 2019;20:386–96. doi: 10.1038/s41590-019-0351-z. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MS, Taylor M, Balic A, Finney CAM, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–624.:e616. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacífico LGG, Marinho FAV, Fonseca CT, Barsante MM, Pinho V, Sales-Junior PA, et al. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+CD25+Foxp3+ T cells independent of interleukin-10. Infect Immun. 2009;77:98–107. doi: 10.1128/IAI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T celldependent and -independent control of allergic inflammation. Immunity. 2008;29:114–26. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 40.van den Biggelaar AH, van Ree R, Roderigues LC, Lell B, Deelder AM, Kremsner PG, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–7. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 41.McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maizels RM. Regulation of immunity and allergy by helminth parasites. Allergy. 2020;75:524–34. doi: 10.1111/all.13944. [DOI] [PubMed] [Google Scholar]
- 43.van den Biggelaar AHJ, Rodrigues LC, van Ree R, van der Zee JS, Hoeksma-Kruize YCM, Souverijn JHM, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 44.Almeida MCF, Lima GS, Cardoso LS, de Souza RP, Campos RA, Cruz AA, et al. The effect of antihelminthic treatment on subjects with asthma from an endemic area of schistosomiasis: a randomized, double-blinded, and placebo-controlled trial. J Parasitol Res. 2012;2012:296856. doi: 10.1155/2012/296856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. 2018;49:801–18. doi: 10.1016/j.immuni.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan SM, Eichenberger RM, Ruscher R, Giacomin PR, Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020;16:e1008508. doi: 10.1371/journal.ppat.1008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartmann S, Schnoeller C, Dahten A, Avagyan A, Rausch S, Lendner M, et al. Gastrointestinal nematode infection interferes with experimental allergic airway inflammation but not atopic dermatitis. Clin Exp Allergy. 2009;39:1585–96. doi: 10.1111/j.1365-2222.2009.03290.x. [DOI] [PubMed] [Google Scholar]
- 48.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–35. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 49.McSorley HJ, O’Gorman MT, Blair N, Sutherland TE, Filbey KJ, Maizels RM. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. 2012;42:2667–82. doi: 10.1002/eji.201142161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chauché C, Vacca F, Chia SL, Richards J, Gregory WF, Ogunkanbi A, et al. A truncated form of HpARI stabilizes IL-33, amplifying responses to the cytokine. Front Immunol. 2020;11:1363. doi: 10.3389/fimmu.2020.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osbourn M, Soares DC, Vacca F, Cohen ES, Scott IC, Gregory WF, et al. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity. 2017;47:739–51. doi: 10.1016/j.immuni.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vacca F, Chauché C, Jamwal A, Hinchy EC, Heieis G, Webster H, et al. A helminth-derived suppressor of ST2 blocks allergic responses. Elife. 2020;9:e54017. doi: 10.7554/eLife.54017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston CJC, Smyth DJ, Kodali RB, White MPJ, Harcus Y, Filbey KJ, et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun. 2017;8:1741. doi: 10.1038/s41467-017-01886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White MPJ, Smyth DJ, Cook L, Ziegler SF, Levings M, Maizels RM. The parasite cytokine mimic Hp-TGM potently replicates the regulatory effects of TGF-β on murine CD4+ T cells. Immunol Cell Biol. 2021;99:848–64. doi: 10.1111/imcb.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smyth DJ, Harcus Y, White MPJ, Gregory WF, Nahler J, Stephens I, et al. TGF-β mimic proteins form an extended gene family in the murine parasite Heligmosomoides polygyrus. Int J Parasitol. 2018;48:379–85. doi: 10.1016/j.ijpara.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook L, Reid KT, Häkkinen E, de Bie B, Tanaka S, Smyth DJ, et al. Induction of stable human FOXP3+ T regs by a parasite-derived TGFβ mimic. Immunol Cell Biol. 2021;99:833–47. doi: 10.1111/imcb.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McSorley HJ, Blair NF, Smith KA, McKenzie ANJ, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–78. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Kleer IM, Kool M, de Bruijn MJW, Willart M, van Moorleghem J, Schuijs MJ, et al. Perinatal activation of the Interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity. 2016;45:1285–98. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 59.Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. 2013;14:364–71. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haspeslagh E, Debeuf N, Hammad H, Lambrecht BN. Murine models of allergic asthma. Methods Mol Biol. 2017;1559:121–36. doi: 10.1007/978-1-4939-6786-5_10. [DOI] [PubMed] [Google Scholar]
- 61.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4’CD25 naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. TGF-β induces a regulatory phenotype in CD4+-CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 63.Smits HH, Hammad H, van Nimwegen M, Soullie T, Willart MA, Lievers E, et al. Protective effect of Schistosoma mansoni infection on allergic asthma depends on intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–40. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 64.McSorley HJ, Chaye MAM, Smits HH. Worms: pernicious parasites or allies against allergies? Parasite Immunol. 2019;41:e12574. doi: 10.1111/pim.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–32. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Fleming JO, Weinstock JV. Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol. 2015;37:277–92. doi: 10.1111/pim.12175. [DOI] [PubMed] [Google Scholar]
- 67.Helmby H. Human helminth therapy to treat inflammatory disorders—where do we stand? BMC Immunol. 2015;16:12. doi: 10.1186/s12865-015-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakanishi W, Yamaguchi S, Matsuda A, Suzukawa M, Shibui A, Nambu A, et al. IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One. 2013;81:e78099. doi: 10.1371/journal.pone.0078099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drake LY, Kita H. IL-33: biological properties, functions, and roles in airway disease. Immunol Rev. 2017;278:173–84. doi: 10.1111/imr.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50:975–91. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–95. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 72.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–41. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 73.Hsu CL, Bryce PJ. Inducible IL-33 expression by mast cells is regulated by a calcium-dependent pathway. J Immunol. 2012;189:3421–9. doi: 10.4049/jimmunol.1201224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardman CS, Panova V, McKenzie ANJ. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43:488–98. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44 (hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 77.Nagarkar DR, Ramirez-Carrozzi V, Choy DF, Lee K, Soriano R, Jia G, et al. IL-13 mediates IL-33-dependent mast cell and type 2 innate lymphoid cell effects on bronchial epithelial cells. J Allergy Clin Immunol. 2015;136:202–5. doi: 10.1016/j.jaci.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 78.Ndaw VS, Abebayehu D, Spence AJ, Paez PA, Kolawole EM, Taruselli MT, et al. TGF-β1 suppresses IL-33-induced mast cell function. J Immunol. 2017;199:866–73. doi: 10.4049/jimmunol.1601983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–47. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–54. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dell’Aringa M, Reinhardt RL. Using cytokine reporter mice to visualize type-2 immunity in vivo. Methods Mol Biol. 2018;1799:211–23. doi: 10.1007/978-1-4939-7896-0_16. [DOI] [PubMed] [Google Scholar]
- 82.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, et al. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–71. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Reese TA, Liang HE, Tager AM, Luster AD, van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perona-Wright G, Mohrs K, Mayer KD, Mohrs M. Differential regulation of IL-4Rα expression by antigen versus cytokine stimulation characterizes Th2 progression in vivo. J Immunol. 2010;184:615–23. doi: 10.4049/jimmunol.0902408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atsuta J, Fujisawa T, Iguchi K, Terada A, Kamiya H, Sakurai M. Inhibitory effect of transforming growth factor β 1 on cytokine-enhanced eosinophil survival and degranulation. Int Arch Allergy Immunol. 1995;108(Suppl 1):31–5. doi: 10.1159/000237197. [DOI] [PubMed] [Google Scholar]
- 90.Sillaber C, Geissler K, Scherrer R, Kaltenbrunner R, Bettelheim P, Lechner K, et al. Type beta transforming growth factors promote interleukin-3 (IL-3)-dependent differentiation of human basophils but inhibit IL-3-dependent differentiation of human eosinophils. Blood. 1992;80:634–41. [PubMed] [Google Scholar]
- 91.Luttmann W, Franz P, Schmidt S, Barth J, Matthys H, Virchow JC., Jr Inhibition of HLA-DR expression on activated human blood eosinophils by transforming growth factor-β1. Scand J Immunol. 1998;48:667–71. doi: 10.1046/j.1365-3083.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 92.Nagase H, Miyamasu M, Yamaguchi M, Kawasaki H, Ohta K, Yamamoto K, et al. Glucocorticoids preferentially upregulate functional CXCR4 expression in eosinophils. J Allergy Clin Immunol. 2000;106:1132–9. doi: 10.1067/mai.2000.110923. [DOI] [PubMed] [Google Scholar]
- 93.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–41. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.