Figure 5.
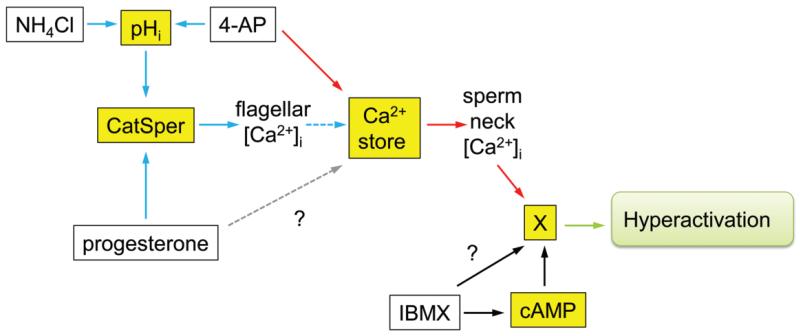
Model of the calcium-signalling cascade in the human spermatozoon: key points in the pathway affected in sub-fertile men (adapted from Barratt and Publicover, 2012). White boxes show compounds used to manipulate pHi (NH4Cl, 4AP), stored calcium (4AP) and cAMP (IBMX). Yellow boxes show signalling components activated by these compounds (pHi, Ca2+, cAMP). Blue and red arrows show Ca2+-signalling pathways involving CatSper and the calcium store, respectively. The Ca2+-store pathway may involve activation of SOCs (not shown; Lefievre et al., 2012). Dashed blue arrow shows the mobilization of stored Ca2+ downstream of CatSper activation by CICR. This occurs in a minority of the cells, is sensitive to modulation (e.g. by capacitation) and is responsible for CatSper-mediated hyperactivation. Dashed grey arrow shows possible modulation of Ca2+-store mobilization by progesterone through CatSper-independent mechanisms (Sagare-Patil et al., 2012). X represents unknown target mechanism/pathway whereby following mobilization of stored Ca2+ hyperactivation is stimulated.? represents potential pathway requiring further experimentation. The amplitude of the calcium transient (stimulated by both 4AP and progesterone) and the hyperactivation response (to 4AP) were significantly related to IVF fertilization rates suggesting the occurrence of important abnormalities in the Ca2+-signalling pathways mediated by CatSper (blue) and the calcium store (red). The calcium signal (4AP and progesterone) in the ICSI patients was significantly lower than in the donors or the IVF patients (≈25%; Fig. 2), providing further evidence of abnormalities in the CatSper functioning/operating complex and calcium store in male infertility. Examination of individual cases demonstrated ≈10% of men undergoing IVF had defective calcium hyperactivation. Although the data are limited, a number of these men did not show a hyperactivation response to IBMX (probably through cAMP, black arrows,?). Previous studies have indicated that, in humans, increases in cAMP do not lead to changes in [Ca2+]i (Brenker et al., 2012), thus these men may suffer from a specific defect in hyperactivation as opposed to a Ca2+-signalling deficit (Supplementary data, Table SII).