Summary
Bloodstream form Trypanosoma brucei acquire iron by receptor-mediated endocytosis of host transferrin. However, the mechanism(s) by which iron is then transferred from the lysosome to the cytosol are unresolved. Here, we provide evidence for the involvement of a protein (TbMLP) orthologous to the mammalian endolysosomal cation channel mucolipin 1. In T. brucei, we show that this protein is localised to the single parasite lysosome. TbMLP null mutants could only be generated in the presence of an expressed ectopic copy, suggesting that the protein is essential. RNAi-mediated ablation resulted in a growth defect in vitro and led to a 7-fold increase in susceptibility to the iron-chelators deferoxamine and salicylhydroxamic acid. Conditional null mutants remained viable when the ectopic copy was repressed, but were hypersensitive to deferoxamine and displayed a growth defect similar to that observed following RNAi. The conditional nulls also retained virulence in vivo in the absence of the doxycycline inducer. These data provide strong evidence that TbMLP has a role in import of iron into the cytosol of African trypanosomes. They also indicate that even when expression is greatly reduced, there is sufficient protein, or an alternative mechanism, to provide the parasite with an adequate supply of cytosolic iron.
Keywords: trypanosome iron transport, lysosome, endocytosis, transferrin
Introduction
Iron is the Earth’s most abundant metal and is required for the growth and replication of almost all organisms. It is an essential component of many enzymes involved in energy metabolism as haem or iron-sulphur clusters due to its versatile redox chemistry. It is also necessary for DNA synthesis as it acts both as a co-factor for ribonucleotide reductase and as part of an iron-sulphur cluster in eukaryotic DNA polymerases (Netz et al., 2012). Iron is of such importance to pathogenic micro-organisms that mammals have developed an iron sequestration response. This is regulated by the innate immune system through IL-6 or IL-22 induced expression of the peptide hormone hepcidin and acts to minimise iron bioavailability (Armitage et al., 2011, Wrighting and Andrews, 2006). Hepcidin- mediated iron deprivation may be crucial for controlling infections with the malaria parasite and a range of bacterial infections (Portugal et al., 2011, Ratledge, 2004, Boelaert et al., 2007, McDermid and Prentice, 2006). This cytokine-mediated iron limiting response is also associated with the anemia seen in animal models of trypanosomiasis and in Nagana (animal African trypanosomiasis) (Stijlemans et al., 2008, Stijlemans et al., 2010a, Stijlemans et al., 2010b). Anemia is one of the major causes of death or morbidity resulting from Nagana, an infection of vital economic importance to livestock-farming in Sub-Saharan Africa.
Bloodstream forms of the African trypanosome Trypanosoma brucei obtain iron from the host iron-carrier protein transferrin. The parasites express a transferrin receptor in their flagellar pocket (FP), encoded by two closely related genes (ESAG6 and ESAG7) which are transcribed from the variant surface glycoprotein expression site (Ligtenberg et al., 1994, Steverding et al., 1994, Steverding et al., 1995, Chaudhri et al., 1994). Expression of the ESAG 6/7 transferrin receptor can be regulated by iron levels. The mechanism involved has not been identified but differs from the mammalian iron-response system (Mussmann et al., 2004, van Luenen et al., 2005, Fast et al., 1999). The ESAG 6/7 heterodimer is attached to the membrane by a single GPI anchor on the ESAG 6 subunit. On binding of transferrin, the receptor/transferrin complex is endocytosed. Iron is then released in the late endosome/lysosome after acidification, the transferrin is proteolytically degraded by a cathepsin L like enzyme (Steverding et al., 2012), and the receptor is recycled back to the flagellar pocket membrane (Pal et al., 2003, Kabiri and Steverding, 2000). However, it is unclear how the released iron is subsequently transported into the cytoplasm for utilisation by the trypanosome. Iron bound to transferrin is in the Fe3+ form, which is practically insoluble (maximal solubility 10−18 M) at physiological pH and temperature. To be exported from the endolysosomal system into the cytoplasm, it must first be reduced to Fe2+.
In mammalian cells, the pathway by which iron gets from endosomes to its final destination has still to be definitively resolved. The route may vary between different cell types, depending on their iron requirements (Sheftel et al., 2007). The best studied mechanism for export of iron from mammalian endosomes is the divalent metal transporter DMT1 (also called SLC11A2, NRAMP2). This protein is required for dietary iron uptake in the gut, and for erythropoiesis as well as normal iron homeostasis (Theil, 2011). However, it is not required for iron uptake in all cell types (Gunshin et al., 2005). Mucolipin 1, (MCOLN1, TRPML1), a member of the transient receptor potential subfamily of ion channels, can also function as an endosomal iron channel in mammals and may facilitate iron release in cells where DMT1 is not expressed (Dong et al., 2008). Mutations within the human MCOLN1 gene can lead to type IV mucolipidosis (MLIV), a lysosomal storage disease characterised by psychomotor retardation, corneal clouding, retinal degeneration and often iron-deficiency or clinical anaemia (Altarescu et al., 2002). MLIV cells have enlarged lysosomes characterised by elevated levels of iron and zinc, as well as lipofuscin (Dong et al., 2008, Eichelsdoerfer et al., 2010). MCOLN1 is also permeable to Ca2+ and is thought to play a role in regulation of membrane trafficking events via PI(3,5)P2-mediated activation of Ca2+ release (Dong et al., 2010). It contains a consensus active site motif for serine lipase (GYSDG), which is responsible for the membrane remodelling activity of MCOLN1 that regulates the formation of tubulovesicular endomembrane structures and lysosomal exocytosis (LaPlante et al., 2011). Thus, the mammalian MCOLN1 protein is bifunctional. MCOLN2 and 3 lack the serine lipase domain, but are otherwise conserved, and have not been implicated in mucolipidosis.
DMT1 orthologues appear to be absent from the trypanosomatid genomes, and endosomal iron transport must be mediated by an alternative route. We report here the identification of a trypanosomal orthologue of the MCOLNs, TbMLP (Mucolipin-like protein). The protein is confined to the endomembrane system, and appears to play a major role in iron metabolism within the parasite.
Results
Identification of an orthologue of Mucolipin 1 in the trypanosomatid genomes
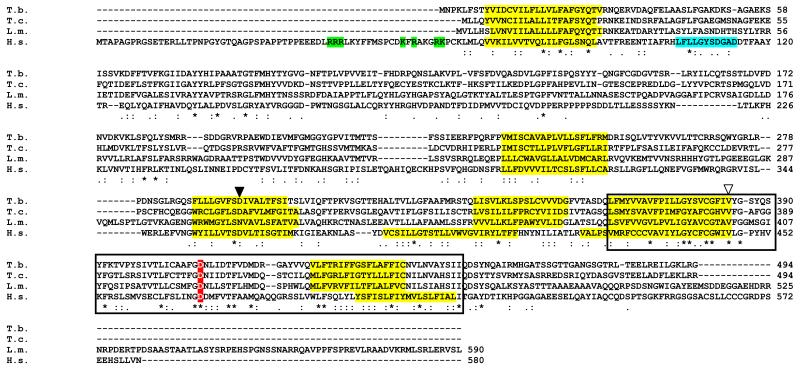
The human MCOLN1 sequence (NP_065394.1) was used to interrogate the trypanosomatid genome data (Berriman et al., 2005, El-Sayed et al., 2005, Ivens et al., 2005, Aslett et al., 2010, Hertz-Fowler et al., 2004). Orthologous sequences were identified in all the trypanosomatids, including the agents of Chagas disease (Trypanosoma cruzi) and the leishmaniases (L. major, L. infantum). Intriguingly, in Leishmania braziliensis, the orthologue is a pseudogene in which the middle portion of the coding sequence has been deleted (annotated as LbrM26_V2.1000 http://tritrypdb.org/), although an open reading frame (ORF) is preserved. The extant ORF is either non-functional or may have another role in L. braziliensis. The T. brucei gene (Tb927.7.950) has an ORF of 1482 bp. The encoded protein has six predicted integral transmembrane domains (identified using PSORT at http://psort.hgc.jp/form2.html), a large luminal loop between transmembrane domains 1 and 2, a putative pore domain between transmembrane domains 5 and 6 and shares similar hydropathy profiles to MCOLN1. Several critical residues in transmembrane helices 5 and 6 and the pore domain, are conserved, including some which are mutated in MLIV patients (Fig.1, Altarescu et al., 2002). Conserved residues were largely restricted to the carboxyl terminal pore domain encompassing transmembrane domains 5 and 6, with the amino terminal domain being poorly conserved. The serine lipase active site of MCOLN1 (consensus motif: ([LIV] {KG} [LIVFY] [LIVMST] G [HYWV] S {YAG} G [GSTAC]) (cyan, Fig. 1) is absent from the first luminal loop suggesting that the kinetoplastid orthologue could be a monofunctional cation channel that lacks membrane remodelling capability. The kinetoplastid proteins also lack the amino terminal cytoplasmic polybasic region implicated in PI(3,5)P2 binding in mammalian MCOLN1 (indicated in green, Fig.1) (Dong et al., 2010) .
Fig. 1.
Alignment of trypanosomatid mucolipin orthologues with human MCOLN1. The putative transmembrane regions (yellow), PI(3,5)P2 binding (green) or serine lipase active site (cyan) (LaPlante et al., 2011) are all highlighted. The boxed region represents the cation channel domain. The white triangle corresponds to a V→L alteration seen in MLIV patient 2 (Altarescu et al., 2002), while the black triangle relates to D→Y change that abolishes Fe2+ transport in mammalian cells (Dong et al., 2008). A transgene carrying the  →K mutation cannot complement MLIV cells (Pryor et al., 2006). Identifiers: Tb – Trypanosoma brucei Tb927.7.950, Tc – Trypanosoma cruzi TcCLB.508215.6, Lm – Leishmania major LmjF.26.0990, Hs – Homo sapiens NP_065394.1, (kinetoplastid genes identified with TriTrypDb/GeneDb codes, mammalian proteins with NCBI locus numbers). Asterisks indicate conserved residues. Alignment was carried out using ClustalW2 at http://www.ebi.ac.uk/Tools/msa/clustalw2/.
→K mutation cannot complement MLIV cells (Pryor et al., 2006). Identifiers: Tb – Trypanosoma brucei Tb927.7.950, Tc – Trypanosoma cruzi TcCLB.508215.6, Lm – Leishmania major LmjF.26.0990, Hs – Homo sapiens NP_065394.1, (kinetoplastid genes identified with TriTrypDb/GeneDb codes, mammalian proteins with NCBI locus numbers). Asterisks indicate conserved residues. Alignment was carried out using ClustalW2 at http://www.ebi.ac.uk/Tools/msa/clustalw2/.
TbMLP is localised to the endolysosomal system and is concentrated in the lysosome
The TbMLP transcript is constitutively expressed in both bloodstream and procyclic T. brucei (Supplementary Fig. 1), consistent with the observation that trypanosomes lack haem-oxygenase and both life-cycle stages require non-haem iron for survival and replication. To identify the subcellular location of the protein, we integrated an epitope tag into the endogenous gene (Experimental Procedures). The final 933 bp of the TbMLP ORF was cloned in frame with 12 copies of a c-myc derived epitope tag (Alsford and Horn, 2008). The tagged protein was localised by immunofluorescence with monoclonal antibody 9E10 directed against the c-myc epitope. This revealed that the protein was limited to vesicular structures occurring between the flagellar pocket/kinetoplast and the nucleus, suggestive of the endolysosomal compartment (Fig. 2A). The antibody recognised a single band only in the transformed cells and not in the wild type on a western blot indicating the specificity (Fig. 2B, lane TbMLP-myc). As a marker for the lysosome, the p67 lysosomal glycoprotein gene was tagged with an influenza HA epitope by in-situ integration using the vector p2708 (Kelly et al., 2007, Kelley et al., 1999). In dual tagged cells, there was almost complete co-localisation between TbMLP and p67 (Fig. 2C and D, where D is a magnification of the boxed region in 2C) indicating that TbMLP was located primarily in the lysosomal compartment of the endocytic pathway. Again the antibody recognised a specific protein only in cells transformed with the p67-HA construct (Fig. 2E, lane 3).
Fig. 2.
Localisation of carboxyl terminally c-myc-tagged TbMLP (TbMLP-myc) in bloodstream form trypanosomes. A. TbMLP-myc was localised with monoclonal antibody 9E10 and is shown as green; red indicates the nucleus (N) and kinetoplast (K) stained with DAPI. TbMLP is concentrated in a vesicular structure between nucleus and kinetoplast with some lighter staining around the flagellar pocket. White bar represents 5 μm. B. Western blot to show that antibody 9E10 recognises a specific band in TbMLP-myc transformed cells; WT indicates wild type. C. TbMLP-myc co-localises with lysosomal glycoprotein p67. To confirm lysosomal localisation of TbMLP-myc the lysosomal marker protein p67 was also tagged with the HA epitope (p67-HA). TbMLP-myc localisation is shown in green, p67-HA in red and DAPI stained DNA in cyan. White bar represents 5 μm. D. Magnification of the region in the dotted box to better show the co-localisation of TbMLP-myc. E. Western blot to show the specificity of the anti-HA antibody for p67 (lane 1: wild type, 2: trypanosomes transformed with TbMLP-myc, 3: trypanosomes transformed with TbMLP-myc and p67-HA. The lower panel shows Coomassie stained gel to show equivalent loading.
RNAi-mediated knockdown of TbMLP results in increased susceptibility to iron-chelators
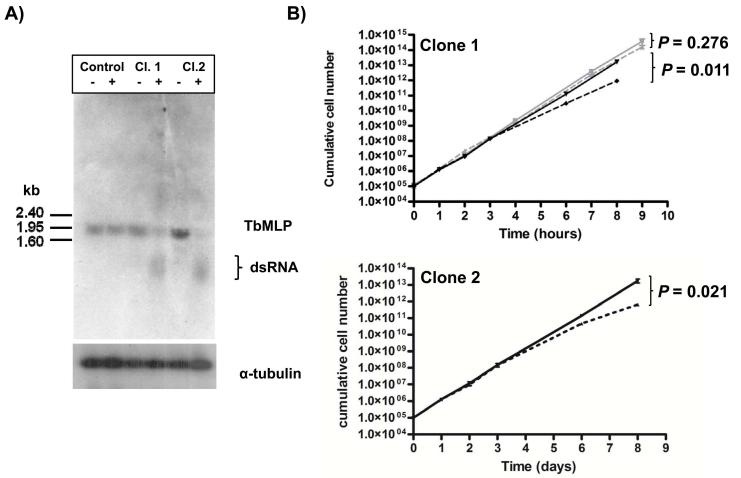
T. brucei bloodstream form cells were transformed with an inducible RNAi construct targeted against the TbMLP mRNA (Experimental Procedures). Expression of the “hairpin” transcript was induced by addition of tetracycline (1 μg ml−1). The ~ 2kb TbMLP mRNA was substantially depleted within 24 hours (Fig. 3A) Quantification by phosphor imaging showed that the transcript had been knocked down between 70% (clone 1) and 85% (clone 2) (Fig. 3A). After three days, growth of the induced cells began to slow, in comparison to the uninduced parasites (Fig. 3B black lines). To control for any effect of tetracycline the growth of the parental cell line was also followed in the presence and absence of inducer (Fig. 3B top panel grey lines). There was no change in growth rates for the parental cells. The difference in growth in the TbMLP RNAi cell lines was shown to be statistically significant (P values for final time point indicated on Fig. 3B). There was no indication of cell death, or changes in morphology or motility.
Fig. 3.
RNAi-mediated depletion of TbMLP has a delayed effect on growth of bloodstream form parasites A. Northern blot showing loss of the TbMLP transcript on induction of RNAi. Cultures of the parental cell line (2T1) and two clones transformed with the RNAi construct were split in two. One flask of each had tetracycline added (1 μg ml−1, indicated by +). The other did not (indicated by −). After 24 hours of induction, total RNA was extracted, blotted and hybridised with the TbMLP ORF. α-tubulin mRNA is shown as loading control. Relative levels of TbMLP mRNA were quanitified by phosphorimager analysis and the ratio between the induced and uninduced populations was calculated (see box below tubulin blot). All lanes were noemalised to the tubulin control. B. Growth curves of RNAi clones in the presence (broken black line) or absence (solid black line) of tetracycline. Cells were seeded at 105 ml−1 and counted after 24 hours. They were then diluted back to 105 ml−1 every 24 hours to maintain exponential growth. For longer counting intervals cells were diluted appropriately. Each clone was plated in triplicate and each well counted twice. P values for the final time point are indicated to the right of the curves. The growth of the parental inducible cell line used to generate the RNAi transfectatnts is shown in grey (solid line without tetracycline, dashed line with tetrcaycline).
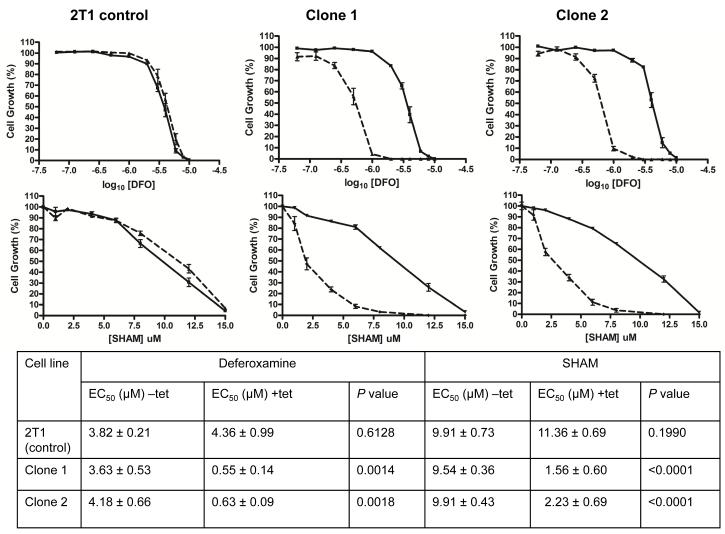
To investigate if TbMLP is involved in iron acquisition, we tested the susceptibility of these trypanosomes to iron-chelators following induction of the RNAi response. We used two chelators, the bacterial siderophore deferoxamine, which is used clinically in the treatment of iron overload syndromes, and salicylhydroxamic acid (SHAM), which has been shown to specifically inhibit the trypanosome alternative oxidase, a mitochondrial di-iron protein essential to the bloodstream form (Chaudhuri et al., 2006, Clarkson et al., 1989, Evans and Brown, 1973). Cells were induced with tetracycline for 24 hours, incubated with the chelators at a range of concentrations and grown for 3 days (Experimental Procedures). Two RNAi clones (derived from separate transfections) examined in parallel were found to be approximately 7-fold more susceptible to SHAM and deferoxamine (Fig. 4), suggesting restricted iron availability when TbMLP1 expression is reduced. The EC50 obtained for deferoxamine for the 2T1 controls was similar to that previously noted for T. brucei strain S427 bloodstream forms (Breidbach et al., 2002).
Fig. 4.
TbMLP down-regulation leads to enhanced susceptibilty to iron chelators. Cells were induced for 24 hours and then plated in varying concentrations of deferoxamine (DFO) or SHAM. Plates were incubated for 72 hours and growth measured using alamarBlue™. The baseline was calculated using alamarBlue™ incubated with medium alone. Control cells (2T1 strain) are shown in the left hand panels and two independent RNAi clones in the centre and right hand panels. Solid line indicates cells incubated without tetracycline (−tet) and broken line indicates cells induced with tetracycline (+tet). EC50 values (+/−SEM) are illustrated in the table. EC50 values were determined using Graphpad PRISM software and the significance of the difference between EC50 values (+/−tet) was calculated using the F-test (P-values shown in table).
Since iron is potentially toxic, most organisms strictly control its uptake. In T. brucei, the transcripts for the transferrin receptor (ESAG6/7) are rapidly up-regulated in response to iron deficiency (Fast et al., 1999). We investigated if TbMLP expression was also subject to up-regulation. When trypanosomes were cultured in conditions known to induce accumulation of the ESAG6/7 mRNA (25 μM deferoxamine for 24 hours), the level of ESAG6/7 mRNA increased by approximately 8-fold (Supplementary Fig. 2A). However, the TbMLP mRNA levels remained constant following this treatment, suggesting that TbMLP is unlikely to be responsive to the same regulatory mechanism as ESAG6/7. We also found that the effects of RNAi-mediated knockdown of TbMLP mRNA were insufficient to promote up-regulation of the transferrin receptor mRNA (Supplementary Fig. 2B). These results could reflect that while deferoxamine treatment results in accumulation of Fe3+:deferoxamine complexes in the lysosome, down-regulation of TbMLP would tend to create an accumulation of “free” Fe2+ since the cognate lysosomal reductase should still be active.
The TbMLP gene can only be deleted in the presence of an expressed ectopic copy
As induction of RNAi produced a relatively minor growth defect, we attempted to generate TbMLP null mutants to determine if there was redundancy in the mechanisms that trypanosomes use for iron acquisition. Targeting vectors were constructed to delete both copies of TbMLP and replace them with blasticidin (BLA) and puromycin (PAC) resistance cassettes. Replacement of the first allele was straightforward; however we were unable to delete the second. On occasions where dual-resistant parasites were selected, we found that this had resulted from integration at alternative sites in the genome (data not shown). These outcomes are generally taken as being indicative of an essential gene. To confirm this, we generated a conditional null mutant by inserting a tetracycline-inducible ectopic copy of TbMLP into TbMLP+/− heterozygote parasites and then attempted to delete the second TbMLP allele. For this experiment, we utilised the T. brucei 2T1 cell line which contains constitutively expressed tetracycline repressor and a tagged rRNA locus suitable for inducible expression (Alsford and Horn, 2008, Alsford et al., 2005). The first TbMLP allele was deleted in the 2T1 cell line using the BLA construct. An inducible copy of TbMLP was then integrated into the tagged rRNA locus. Expression was shown to be inducible and repressible, with silencing of the transgene occurring within 24 hours of tetracycline withdrawal (Fig. 5B lane SKO). Finally, the second TbMLP allele was deleted, with transformants maintained on tetracycline to promote expression of the ectopic copy. Clones in which both endogenous alleles had been deleted were readily obtained (Fig. 5A lanes 1,2 and 3).
Fig. 5.
TbMLP can be deleted in the presence of a regulated ectopic copy. A. Deletion of endogenous TbMLP alleles. Map shows location of HindIII sites and the small black line indicates the probe. The Southern blot of genomic DNA digested with HindIII shows interruption of the two endogenous alleles in the conditional null mutants (lanes 1, 2 and 3 are individual clones, the wild type (WT) and the heterozygote (SKO) are shown for comparison). B. Repression of conditional TbMLP expression leads to down-regulation within 24 hours. Total RNA was isolated and blotted from wild type (WT), heterozygote (SKO) and 2 conditional null clones (DKO1 and DKO2), +tet, cells incubated with tetracycline (1 μg ml−1), −tet, cells washed (× 3) and removed from tetracycline for 24 hours. C. Growth phenotype of conditional null mutant recapitulates the RNAi phenotype. The broken black line in each case indicates the cumulative growth of the cell line maintained on tetracycline. The solid black line indicates the growth of the same line after removal of tetracycline by washing. A representative experiment is shown for each clone.
Following withdrawal of tetracycline from the cultured conditional null mutants, the TbMLP mRNA signal was virtually undetectable by 24 hours (Fig. 5B lanes DKO1 −tet and DKO2 −tet)). Quantitative RT-PCR analysis of these RNA samples indicated a drop of between 2500 and 3500 fold in the transcript levels for MLP between the induced and repressed states (see supplementary tables 1 and 2). However, we observed only a mild detrimental effect on growth, which became apparent after 4-6 days (Fig. 5C). This phenotype was soon masked by the outgrowth of cells which had returned to the normal growth rate. This suggests there may be an alternative mechanism available to complement for the loss of TbMLP. Analysis of one of the conditional cell lines which had returned to the normal growth rate revealed a recombination event that had deleted a copy of the tet repressor along with its phleomycin resistance cassette (data not shown). This clone had taken over the population since no phleomycin resistant cells could be recovered. Reversion events such as this are commonly seen with conditional knockouts of essential genes in trypanosomes (Krieger et al., 2000).
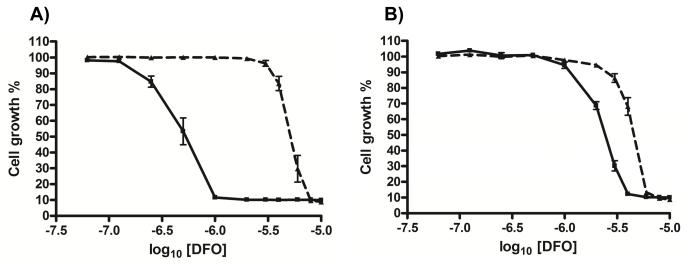
The conditional null mutants were also investigated for susceptibility to iron-chelators. As with the RNAi cells, the null mutants displayed increased sensitivity to the growth inhibitory effects of deferoxamine when TbMLP expression was down-regulated. Of the two conditional null mutant clones tested, one was 11-fold more susceptible and the other 2-fold when tetracycline was withdrawn (Fig. 6). The variation between clones could be due to the presence of escape mutants/transcriptional leakiness. Nevertheless, it is clear that down-regulation of TbMLP expression, either in an RNAi or conditional null mutant background, results in increased sensitivity to iron chelating agents. Deferoxamine has a high specificity for iron and the effects observed are unlikely to be due to chelation of other biologically important transition metal ions.
Fig. 6.
Conditional null mutants also display enhanced susceptibility to deferoxamine (DFO). Cells were repressed for 24 hours and then plated in varying concentrations of deferoxamine. Control cells were maintained in tetracycline (1 μg ml−1). Plates were incubated for 72 hours and growth measured using alamarBlue™ as in Fig. 4. The broken black lines represent growth in the presence of tetracycline. The solid black lines represent growth in the absence of tetracycline. A. DKO 1 (EC50 +tet 5.11 ± 0.57 μM, −tet 0.48 ± 0.11 μM; P<0.0002), B. DKO2. (EC50 +tet 4.35 ± 0.27 μM, −tet 2.28 ± 0.27 μM; P<0.0016). EC50 values were calculated using Graphpad PRISM software and the significance of the difference between EC50 values (+/−tet for each clone) was calculated using the F-test.
Depletion of TbMLP results in increased susceptibility to superoxide
One consequence of depletion in intracellular iron levels should be a concomitant decrease in the activities of iron dependent enzymes. We had already seen an indication of this when the induction of RNAi against TbMLP resulted in an enhanced susceptibility to SHAM suggesting a decreased level of alternative oxidase activity. This enzyme is the primary target of SHAM (Opperdoes et al., 1976, Evans and Brown, 1973, Ott et al., 2006, Helfert et al., 2001, Brohn and Clarkson, 1978, Clarkson et al., 1989). To further test this, we subjected both the conditional null mutants and the RNAi cells to the superoxide generator paraquat (methyl viologen). Trypanosomes express only iron-dependent superoxide dismutases (Fe-SODs) (Dufernez et al., 2006, Wilkinson et al., 2006). RNAi mediated ablation of one of these (TbSOD A) resulted in enhanced susceptibility to paraquat in bloodstream form T. brucei (Wilkinson et al., 2006). We reasoned therefore, that if depletion of TbMLP did result in a lower availability of intracellular iron, then the activity of TbSOD A should be reduced and hence the cells should become more susceptible to paraquat.
Incubation of the conditional null mutants in the absence of tetracycline, which leads to down-regulation of TbMLP, resulted in greater sensitivity to paraquat (Fig. 7). A similar trend was observed in the RNAi cell line. In this case, addition of tetracycline led to reduced expression and a concomitant increase in paraquat susceptibility (Fig. 7). The finding of similar results in both RNAi and conditional null mutants excludes the possibility of an antioxidant effect of tetracycline itself on paraquat susceptibility.
Fig. 7.
Parasites with reduced levels of TbMLP exhibit enhanced susceptibility to superoxide. Expression of TbMLP in bloodstream form T. brucei was repressed (conditional null cells −tet) or induced (RNAi cells +tet) for 48 hours and cells were then plated in varying concentrations of paraquat. Plates were incubated for 72 hours and growth measured using alamarBlue™. EC50 values were calculated using variable slope sigmoidal curve fit (Graphpad PRISM software) and the significance of the difference between EC50 values (+/−tet for each clone) was calculated using the F-test (P-values indicated). Lanes D) DKO (EC50 −tet 4.33 ± 0.34 μM, +tet 5.62 ± 0.30 μM), Lanes R) RNAi line. (EC50 −tet 5.14 ± 0.18 μM, +tet 4.72 ± 0.12 μM)
Depletion of TbMLP does not affect virulence in vivo
To test whether the extent to which expression of TbMLP is required for virulence, we infected female Balb/c mice with the conditional mutant. The parental strain, T. brucei S427, is monomorphic and causes an acute and rapidly lethal infection in mice. One group of five mice was given doxycycline (200 μg ml−1) in their drinking water to maintain expression of the transgene. The other group was maintained in the absence of the inducer, so that expression should be repressed. Mice were infected with only 500 trypanosomes to minimise the chance of an escape mutant being present in the initial inoculum. Trypanosomes in the repressed group were taken off tetracycline for 48 hours prior to infection to ensure minimal expression of TbMLP. All mice showed patent parasitemia by 3 days post-infection and had to be culled due to high parasitemia on day 5. Examination of blood smears revealed no significant differences in parasitemia between the groups (Fig. 8).
Fig. 8.
Conditional null mutants retain virulence in vivo in the absence of TbMLP induction. Groups of 5 mice were infected with the null mutant cell lines DKO1 and DKO 2 in the presence or absence of doxycycline (Experimental Procedures). The course of infection was followed by tail-blood parasitemia. The chart indicates the number of parasites observed in 10 fields of a Giemsa stained blood smear. The data were analysed with Graphpad PRISM software. For both conditional null mutants there was no significant difference between the course of parasitemia in the presence or absence of inducer (P-values shown above bars). All mice were euthanized after 5 days to prevent in unnecessary suffering in accordance with statutory requirements.
Discussion
Iron is an essential nutrient for almost all pathogenic micro-organisms. Its role in human infectious trypanosomatids has been recently reviewed (Taylor and Kelly, 2010). African trypanosomes obtain iron by uptake of transferrin, the carrier protein that circulates in the host bloodstream. The fate of the endocytosed transferrin protein has been well documented; it is degraded in the parasite lysosome, and the proteolytic fragments are ejected back into the bloodstream (Steverding et al., 1995). However, the mechanism by which the released iron escapes from the lysosome into the parasite cytosol is less clear. Here, we have identified and characterised a T. brucei protein (TbMLP) orthologous to the mucolipin family of ion channels that we propose as a candidate for this role. It is expressed in both bloodstream and insect stages of the parasite and is confined to the endocytic system, with the highest expression being found in the p67 positive compartment corresponding to the single terminal lysosome (Kelley et al., 1999).
Using an RNAi-based approach, we showed that although trypanosomes could initially divide normally when expression of TbMLP had been reduced, growth retardation would occur after 3 days. This is in broad agreement with previous studies which suggested that trypanosomes have an internal iron store capable of supporting 48 hours of cell division (approximately 7 generations) in the absence of exogenous iron supplies (Steverding, 1998, van Luenen et al., 2005). In contrast to this relatively minor growth defect, knockdown of TbMLP did have a significant impact on the susceptibility of trypanosomes to the iron-chelators deferoxamine and SHAM. These compounds exhibit their effects in different parts of the cell. Deferoxamine is largely confined to the endosomal system as it cannot readily cross membranes (Kurz et al., 2006), while SHAM can penetrate to the mitochondrion where it specifically inhibits the di-iron protein trypanosome alternative oxidase (Clarkson et al., 1989, Evans and Brown, 1973, Ott et al., 2006). Thus, the effect seen with TbMLP RNAi suggests a general defect in the iron supply. This phenotype was confirmed in the conditional null mutant (eliminating the possibility of off-target RNAi effects being responsible). In addition, depletion of TbMLP by RNAi or in the conditional null mutants also resulted in a greater susceptibility to the superoxide generator paraquat. Since the SOD repertoire of trypanosomes is entirely dependent on iron for catalytic activity this result gives further evidence that TbMLP plays a significant role in the delivery of iron to the trypanosome cytoplasm for incorporation into metalloenzymes. The effect of TbMLP depletion on paraquat sensitivity was relatively small but reproducible. Two parameters may play a role in the level of sensitivity observed. The first is that SOD itself may have a long half-life thus even when the iron supply is compromised a reasonable level of activity can be maintained for some while as has been demonstrated in trypanosomes treated with deferoxamine (Breidbach et al., 2002). Secondly as iron itself plays a major role in the generation of oxidative stress enhanced by superoxide (the superoxide-driven Fenton reaction (Halliwell and Gutteridge, 2007)) then manipulations which result in a depletion of intracellular labile iron will reduce the level of oxidative stress generated during paraquat administration. This could, to some extent, counterbalance the decrease in SOD activity.
Given the putative topology and the lysosomal location of the protein, our data therefore support a role for TbMLP as the T. brucei endosomal iron-release channel. Unequivocal experimental evidence of transport activity is difficult to achieve in the absence of a heterologous expression system that mimics the trypanosome lysosomal membrane. Our data suggested that TbMLP could be an essential protein since deletion of both alleles was only possible in the presence of an ectopic copy of the gene. However, conditional null mutants could grow in vivo and in vitro in the absence of detectable TbMLP transcripts. Either basal level expression of the transgene is sufficient to meet the needs of the parasite, or there is an alternative pathway for iron uptake. It was noticeable however that there appeared to be strong selection for adaptation as back extrapolation of the outgrowth lines to the X-axis in Fig. 5C indicated that normally growing cells were appearing in the population within 2 days of the removal of tetracycline from the medium. One population had adapted by rearrangement of the tet repressor genes controlling ectopic expression of TbMLP.
We did attempt to measure the labile iron pool in these trypanosomes using a calcein quenching assay (Esposito et al., 2002). The level detected in control cells was close to the limit of sensitivity of the technique (~100 nM), rendering accurate measurement unachievable (Esposito et al., 2002, Kakhlon and Cabantchik, 2002).
It remains possible that the RNAi growth phenotype observed after 3 days could result from a reduced supply of metals other than iron (or a combination), since mammalian MCOLN1 is known to be permeable to Ca2+ and Zn2+ (Dong et al., 2008, Dong et al., 2010, Eichelsdoerfer et al., 2010). In trypanosomes however, the major players in Ca2+ homeostasis are known to be the plasma membrane, mitochondrion and acidocalcisomes, rather than the lysosome (Docampo and Lukes, 2012, Moreno and Docampo, 2003). Significantly, no effect on cell morphology was seen with either the RNAi cell lines or the conditional null mutants. Loss of function mutations in mammalian mucolipin 1 are known to cause gross defects in lysosomal morphology, endocytosis and autophagy. The lack of the serine lipase domain in TbMLP may account for the lack of these phenotypes.
Whether TbMLP is the only means by which iron is able to access the cytosol in T. brucei bloodstream forms remains to be resolved. However, the properties of this protein suggest a major role in the transfer of transition metal ions, notably Fe2+, across the lysosomal membrane and into the cytoplasm. Because parasites must obtain essential nutrients from their hosts, nutrient uptake mechanisms could prove to be an exploitable Achilles’ heel for new therapies.
Experimental Procedures
Parasite culture and genetic manipulation
T. brucei brucei strain Lister 427 bloodstream trypomastigotes were cultured in HMI-9 (Invitrogen) supplemented with 10% v/v tetracycline-free fetal bovine serum (BioSera), penicillin/streptomycin (GibcoBRL) and β–mercaptoethanol (Sigma) at 37°C in a 5% CO2 atmosphere (Hirumi and Hirumi, 1989). T. brucei 2T1 cell lines carrying two copies of the tet repressor gene and a tagged inducible expression locus were cultured as above, but supplemented with phleomycin (1 μg ml−1) and puromycin (1 μg ml−1) (Alsford et al., 2005). RNAi cells were maintained on phleomycin (1 μg ml−1) and hygromycin (0.5 μg ml−1). For the conditional null mutants, cells were maintained on phleomycin (1 μg ml−1), puromycin (1 μg ml−1), blasticidin (10 μg ml−1), hygromycin (0.5 μg ml−1) and tetracycline (1 μg ml−1). Conditional null mutants were not maintained in culture for more than two weeks unless required by the experiment, to avoid selection of escape mutants. Growth curves and drug susceptibility assays were carried out in the absence of selection agents, except for tetracycline as required.
For transfection, parasites (5 × 107) were pelleted and resuspended in 100 μl human T-cell nucleofection buffer (Lonza) and electroporated using program ×.001 on the Nucleofector. The cells were resuspended in 200 ml HMI-9 and allowed to recover for 4-6 hours. The appropriate selective agent was then added (at concentrations mentioned above) and the cells seeded in 48-well plates. Positive clones were isolated after 6 days selection at 37°C.
Constructs
For tagging the endogenous TbMLP gene with a carboxyl terminal c-myc tag, the 3′ 933 bp of TbMLP (Tb927.7.950 at http://tritrypdb.org) was amplified using primers:
F: 5′ -aaaaggcgcgccATTCCATGAGAAGGTCAGATGAC
R: 5′- cccctctagaCCCCCTCAACTTCCCCAATATTT (restriction sites in italics used for cloning). The product was digested with AscI and XbaI and ligated into AscI/XbaI digested pNATx12MYC (a gift from Sam Alsford, LSHTM (Alsford and Horn, 2008)). For transfection, the construct was linearised using the unique SphI site within the TbMLP ORF.
The RNAi construct was based on stem-loop vector pRPaiSL (Alsford and Horn, 2008). A TbMLP gene-internal fragment of 407 bp from nt 661-1067, identified using RNAit software (trypanofan.path.cam.ac.uk/software/RNAit.html), was amplified using primers:
F: 5′- ttttgggcccggtaccGCAGTATCGAGGAGCGTTTC
R: 5′-aaaatctagaggatccAAAACCATCGACCACTACGC
The sense orientation was cloned by digestion with KpnI and BamHI, while the antisense was cloned with ApaI and XbaI, restriction sites indicated by italics.
Targeted gene deletion and construction of conditional null mutant
Gene deletion constructs were generated by cloning the TbMLP 5′ and 3′ flanking sequences into drug resistance cassettes for blasticidin and puromycin. 356 bp of 5′- flanking DNA was amplified and cloned using primers:
F: 5′ - aaaagcggccgcGAATCATGATCAGCGAACCACG
R: 5′- ttttggatccACAGCTTCGGATTCATATGTG
For the 3′- flanking DNA a 333 bp fragment was cloned using primers:
F: 5′ ttttgggcccGGTAGTTTCCTGCCCTTCTTAT
R: 5′ ttttggtaccTGTTTCGACTAGGGTTCGCTGA.
The constructs were digested with NotI and KpnI for transfection.
A tetracycline regulated copy of the TbMLP gene was created by inserting the full length ORF into the vector pRPc6MYCn with a stop codon inserted (Alsford and Horn, 2008).
Immunofluorescence
Exponentially growing parasites were fixed in 2% paraformaldehyde in normal growth medium. The cells were then pelleted and washed in PBS. Fixed cells were dotted onto slides and air-dried. For internal labelling, cells were permeabilised by incubation in 0.5% Triton-X-100/PBS for 20 mins. Slides were washed 3 × in PBS then blocked for 15 mins in 50% FBS/PBS. Primary antibody was added at an appropriate dilution in 20% FBS/PBS and the slides incubated for 1 hour then washed 3 × in PBS. Secondary antibody was added at an appropriate dilution in 20% FBS/PBS and the slides incubated for 45 mins. Slides were washed 3 × in PBS, then mounted in 1:1 PBS:glycerol with DAPI. Slides were examined on a Zeiss Axioplan 510 confocal laser scanning microscope.
RNA interference / Conditional null mutant drug susceptibility assays
For RNAi cell lines, one flask was induced with tetracycline (1 μg ml−1) while the other remained uninduced, as a control. For conditional nulls, the cells were maintained on tetracycline (1 μg ml−1) and prior to the experiment were washed 3 × in 1 volume of medium without tetracycline (estimated final concentration of tetracycline <1 pg ml−1). The population was then split in two and tetracycline added back to the control population. After 24 hours of induction/repression, the cells were seeded into 96-well plates at 104 ml−1. For paraquat assays the induction/repression was prolonged to 48 hours prior to drug testing to allow for depletion of Fe-SOD activity mediated by loss of TbMLP dependent iron transport. The appropriate drug concentration was added to each well and the plates incubated at 37°C for 2 days. 20 μl alamarBlue™ was then added to each well and the plates incubated at 37°C overnight. Fluorescence was read in a Gemini Fluorimeter at λex 530 nm and λem 585 nm with a cut-off set at 570 nm (Molecular Devices).
cDNA synthesis and qPCR quantification of MLP mRNA levels in conditional null mutant
cDNA was synthesised using the Superscript VILO cDNA synthesis kit (Invitrogen). For each RNA sample three separate cDNA syntheses were carried out. Briefly 1μg total RNA was reverse transcribed for 2 hrs at 42°C. The reaction was terminated at 85°C for 5 mins. Each was taken forward into the qPCR reaction. cDNA equivalent to 100ng was amplified using primers:
MLP QF 5′-ACATACCGACTGCAGCAACTGG and
MLP QR 5′-CCTAGTACATCGCTTGCTTGA, giving a product of 169 bp
TERT QF 5′-GAGCGTGTGACTTCCGAAGG and
TERT QR 5′-AGGAACTGTCACGGAGTTTGC giving a product of 108 bp.
The reaction was carried out using the QuantiTect® SYBR® Green kit (Qiagen) in a Rotor-gene 3000 instrument (Corbett Research). Cycling conditions were: 1 cycle of 95°C for 15 mins followed by 40 cycles of 94°C for 15 s, 58°C for 20s and 72°C for 30 s, Data acquisition was carried out after each 72°C stage and the products were analysed by a melt curve after the final cycle.
The reference transcript for normalisation was TbTERT (telomerase reverse transcriptase) as this had previously been validated in a variety of conditions (Brenndorfer and Boshart, 2010). A standard curve was derived by amplification of a series of 10-fold dilutions of the target PCR products.
For RNAi, knockdown was quantified by phosphorimager analysis of Northern blots probed with the TbMLP ORF (test probe) and β-tubulin (internal control for normalisation of loading). Analysis was performed using a Typhoon imager and ImageQuant software (GE Healthcare Life Sciences). Phosphorimager analysis was used rather than qPCR to ensure that only full-length mRNA was quantified.
In vivo infection
Conditional null mutants maintained on tetracycline, were washed 3 × in HMI-9 and split into two flasks. One flask had tetracycline (1 μg ml−1) added back. The flasks were incubated for 48 hours to ensure repression of the transgene. Female Balb/c mice were infected with 500 bloodstream trypomastigotes i.v. Five mice were infected in each group. Mice which received the tetracycline induced cells had doxycycline (200 μg ml−1) in their drinking water with 5% sucrose. The other mice were given 5% sucrose. Doxycycline treatment was started 24 hours prior to infection. Parasitemia was monitored by tail bleeds and mice were euthanized when a high parasitemia (non-recoverable) was apparent, in accordance with statutory animal welfare requirements and UK Home Office regulations.
Supplementary Material
Acknowledgements
This work was supported by the Wellcome Trust (grant 084175 to J.M.K). We would like to thank Sam Alsford, David Horn and Mark Carrington for vectors, Shane Wilkinson for critical reading of the manuscript and Anne Koerber for help with the figures. We would also like to thank the staff of the genome databases TriTrypDB and GeneDB for sequence data used in this publication.
References
- Alsford S, Horn D. Single-locus targeting constructs for reliable regulated RNAi and transgene expression in Trypanosoma brucei. Mol Biochem Parasitol. 2008;161:76–79. doi: 10.1016/j.molbiopara.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsford S, Kawahara T, Glover L, Horn D. Tagging a T. brucei RRNA locus improves stable transfection efficiency and circumvents inducible expression position effects. Mol Biochem Parasitol. 2005;144:142–148. doi: 10.1016/j.molbiopara.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarescu G, Sun M, Moore DF, Smith JA, Wiggs EA, Solomon BI, et al. The neurogenetics of mucolipidosis type IV. Neurology. 2002;59:306–313. doi: 10.1212/wnl.59.3.306. [DOI] [PubMed] [Google Scholar]
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, Carrington M, et al. TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 2010;38:D457–462. doi: 10.1093/nar/gkp851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195:1745–1753. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- Breidbach T, Scory S, Krauth-Siegel RL, Steverding D. Growth inhibition of bloodstream forms of Trypanosoma brucei by the iron chelator deferoxamine. Int J Parasitol. 2002;32:473–479. doi: 10.1016/s0020-7519(01)00310-1. [DOI] [PubMed] [Google Scholar]
- Brenndorfer M, Boshart M. Selection of reference genes for mRNA quantification in Trypanosoma brucei. Mol Biochem Parasitol. 2010;172:52–55. doi: 10.1016/j.molbiopara.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Brohn FH, Clarkson AB., Jr. Quantitative effects of salycylhydroxamic acid and glycerol on Trypanosoma brucei glycolysis in vitro and in vivo. Acta Trop. 1978;35:23–33. [PubMed] [Google Scholar]
- Chaudhri M, Steverding D, Kittelberger D, Tjia S, Overath P. Expression of a glycosylphosphatidylinositol-anchored Trypanosoma brucei transferrin-binding protein complex in insect cells. Proc Natl Acad Sci U S A. 1994;91:6443–6447. doi: 10.1073/pnas.91.14.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri M, Ott RD, Hill GC. Trypanosome alternative oxidase: from molecule to function. Trends Parasitol. 2006;22:484–491. doi: 10.1016/j.pt.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Clarkson AB, Jr., Bienen EJ, Pollakis G, Grady RW. Respiration of bloodstream forms of the parasite Trypanosoma brucei brucei is dependent on a plant-like alternative oxidase. J Biol Chem. 1989;264:17770–17776. [PubMed] [Google Scholar]
- Docampo R, Lukes J. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol. 2012;28:31–37. doi: 10.1016/j.pt.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufernez F, Yernaux C, Gerbod D, Noel C, Chauvenet M, Wintjens R, et al. The presence of four iron-containing superoxide dismutase isozymes in trypanosomatidae: characterization, subcellular localization, and phylogenetic origin in Trypanosoma brucei. Free Radic Biol Med. 2006;40:210–225. doi: 10.1016/j.freeradbiomed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Eichelsdoerfer JL, Evans JA, Slaugenhaupt SA, Cuajungco MP. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J Biol Chem. 2010;285:34304–34308. doi: 10.1074/jbc.C110.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- Esposito BP, Epsztejn S, Breuer W, Cabantchik ZI. A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal Biochem. 2002;304:1–18. doi: 10.1006/abio.2002.5611. [DOI] [PubMed] [Google Scholar]
- Evans DA, Brown RC. The inhibitory effects of aromatic hydroxamic acids on the cyanide-insensitive terminal oxidase of Trypanosoma brucei. Trans R Soc Trop Med Hyg. 1973;67:258. doi: 10.1016/0035-9203(73)90160-0. [DOI] [PubMed] [Google Scholar]
- Fast B, Kremp K, Boshart M, Steverding D. Iron-dependent regulation of transferrin receptor expression in Trypanosoma brucei. Biochem J. 1999;342(Pt 3):691–696. [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 2007. [Google Scholar]
- Helfert S, Estevez AM, Bakker B, Michels P, Clayton C. Roles of triosephosphate isomerase and aerobic metabolism in Trypanosoma brucei. Biochem J. 2001;357:117–125. doi: 10.1042/0264-6021:3570117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Fowler C, Peacock CS, Wood V, Aslett M, Kerhornou A, Mooney P, et al. GeneDB: a resource for prokaryotic and eukaryotic organisms. Nucleic Acids Res. 2004;32:D339–343. doi: 10.1093/nar/gkh007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiri M, Steverding D. Studies on the recycling of the transferrin receptor in Trypanosoma brucei using an inducible gene expression system. Eur J Biochem. 2000;267:3309–3314. doi: 10.1046/j.1432-1327.2000.01361.x. [DOI] [PubMed] [Google Scholar]
- Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- Kelley RJ, Alexander DL, Cowan C, Balber AE, Bangs JD. Molecular cloning of p67, a lysosomal membrane glycoprotein from Trypanosoma brucei. Mol Biochem Parasitol. 1999;98:17–28. doi: 10.1016/s0166-6851(98)00155-8. [DOI] [PubMed] [Google Scholar]
- Kelly S, Reed J, Kramer S, Ellis L, Webb H, Sunter J, et al. Functional genomics in Trypanosoma brucei: a collection of vectors for the expression of tagged proteins from endogenous and ectopic gene loci. Mol Biochem Parasitol. 2007;154:103–109. doi: 10.1016/j.molbiopara.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol Microbiol. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- Kurz T, Gustafsson B, Brunk UT. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006;273:3106–3117. doi: 10.1111/j.1742-4658.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- LaPlante JM, Falardeau JL, Brown EM, Slaugenhaupt SA, Vassilev PM. The cation channel mucolipin-1 is a bifunctional protein that facilitates membrane remodeling via its serine lipase domain. Exp Cell Res. 2011;317:691–705. doi: 10.1016/j.yexcr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg MJ, Bitter W, Kieft R, Steverding D, Janssen H, Calafat J, Borst P. Reconstitution of a surface transferrin binding complex in insect form Trypanosoma brucei. EMBO J. 1994;13:2565–2573. doi: 10.1002/j.1460-2075.1994.tb06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermid JM, Prentice AM. Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMP1 (SLC11A1) on host-pathogen interactions in tuberculosis and HIV. Clin Sci (Lond) 2006;110:503–524. doi: 10.1042/CS20050273. [DOI] [PubMed] [Google Scholar]
- Moreno SN, Docampo R. Calcium regulation in protozoan parasites. Curr Opin Microbiol. 2003;6:359–364. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Engstler M, Gerrits H, Kieft R, Toaldo CB, Onderwater J, et al. Factors affecting the level and localization of the transferrin receptor in Trypanosoma brucei. J Biol Chem. 2004;279:40690–40698. doi: 10.1074/jbc.M404697200. [DOI] [PubMed] [Google Scholar]
- Netz DJ, Stith CM, Stumpfig M, Kopf G, Vogel D, Genau HM, et al. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol. 2012;8:125–132. doi: 10.1038/nchembio.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes FR, Aarsen PN, van der Meer C, Borst P. Trypanosoma brucei: an evaluation of salicylhydroxamic acid as a trypanocidal drug. Exp Parasitol. 1976;40:198–205. doi: 10.1016/0014-4894(76)90082-5. [DOI] [PubMed] [Google Scholar]
- Ott R, Chibale K, Anderson S, Chipeleme A, Chaudhuri M, Guerrah A, et al. Novel inhibitors of the trypanosome alternative oxidase inhibit Trypanosoma brucei brucei growth and respiration. Acta Trop. 2006;100:172–184. doi: 10.1016/j.actatropica.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Pal A, Hall BS, Jeffries TR, Field MC. Rab5 and Rab11 mediate transferrin and anti-variant surface glycoprotein antibody recycling in Trypanosoma brucei. Biochem J. 2003;374:443–451. doi: 10.1042/BJ20030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17:732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic. 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C. Iron, mycobacteria and tuberculosis. Tuberculosis (Edinb) 2004;84:110–130. doi: 10.1016/j.tube.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- Steverding D. Bloodstream forms of Trypanosoma brucei require only small amounts of iron for growth. Parasitol Res. 1998;84:59–62. doi: 10.1007/s004360050357. [DOI] [PubMed] [Google Scholar]
- Steverding D, Sexton DW, Wang X, Gehrke SS, Wagner GK, Caffrey CR. Trypanosoma brucei: Chemical evidence that cathepsin L is essential for survival and a relevant drug target. Int J Parasitol. 2012;42:481–488. doi: 10.1016/j.ijpara.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Steverding D, Stierhof YD, Chaudhri M, Ligtenberg M, Schell D, Beck-Sickinger AG, Overath P. ESAG 6 and 7 products of Trypanosoma brucei form a transferrin binding protein complex. Eur J Cell Biol. 1994;64:78–87. [PubMed] [Google Scholar]
- Steverding D, Stierhof YD, Fuchs H, Tauber R, Overath P. Transferrin-binding protein complex is the receptor for transferrin uptake in Trypanosoma brucei. J Cell Biol. 1995;131:1173–1182. doi: 10.1083/jcb.131.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stijlemans B, Vankrunkelsven A, Brys L, Magez S, De Baetselier P. Role of iron homeostasis in trypanosomiasis-associated anemia. Immunobiology. 2008;213:823–835. doi: 10.1016/j.imbio.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Stijlemans B, Vankrunkelsven A, Brys L, Raes G, Magez S, De Baetselier P. Scrutinizing the mechanisms underlying the induction of anemia of inflammation through GPI-mediated modulation of macrophage activation in a model of African trypanosomiasis. Microbes Infect. 2010a;12:389–399. doi: 10.1016/j.micinf.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Stijlemans B, Vankrunkelsven A, Caljon G, Bockstal V, Guilliams M, Bosschaerts T, et al. The central role of macrophages in trypanosomiasis-associated anemia: rationale for therapeutical approaches. Endocr Metab Immune Disord Drug Targets. 2010b;10:71–82. doi: 10.2174/187153010790827966. [DOI] [PubMed] [Google Scholar]
- Taylor MC, Kelly JM. Iron metabolism in trypanosomatids, and its crucial role in infection. Parasitology. 2010;137:899–917. doi: 10.1017/S0031182009991880. [DOI] [PubMed] [Google Scholar]
- Theil EC. Iron homeostasis and nutritional iron deficiency. J Nutr. 2011;141:724S–728S. doi: 10.3945/jn.110.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luenen HG, Kieft R, Mussmann R, Engstler M, ter Riet B, Borst P. Trypanosomes change their transferrin receptor expression to allow effective uptake of host transferrin. Mol Microbiol. 2005;58:151–165. doi: 10.1111/j.1365-2958.2005.04831.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson SR, Prathalingam SR, Taylor MC, Ahmed A, Horn D, Kelly JM. Functional characterisation of the iron superoxide dismutase gene repertoire in Trypanosoma brucei. Free Radic Biol Med. 2006;40:198–209. doi: 10.1016/j.freeradbiomed.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.