Abstract
Background
Endometrial adenocarcinoma (womb cancer) is a malignant growth of the lining (endometrium) of the womb (uterus). It is distinct from sarcomas (tumours of the uterine muscle). Survival depends the risk of microscopic metastases after surgery. Adjuvant (postoperative) chemotherapy improves survival from some other adenocarcinomas, and there is evidence that endometrial cancer is sensitive to cytotoxic therapy. This systematic review examines the effect of chemotherapy on survival after hysterectomy for endometrial cancer.
Objectives
To assess efficacy of adjuvant (postoperative) chemotherapy for endometrial cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2010, Issue 3), MEDLINE and EMBASE up to August 2010, registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) comparing adjuvant chemotherapy with any other adjuvant treatment or no other treatment.
Data collection and analysis
We used a random-effects meta-analysis to assess hazard ratios (HR) for overall and progression-free survival and risk ratios (RR) to compare death rates and site of initial relapse.
Main results
Five RCTs compared no additional treatment with additional chemotherapy after hysterectomy and radiotherapy. Four trials compared platinum based combination chemotherapy directly with radiotherapy. Indiscriminate pooling of survival data from 2197 women shows a significant overall survival advantage from adjuvant chemotherapy (RR (95% CI) = 0.88 (0.79 to 0.99)). Sensitivity analysis focused on trials of modern platinum based chemotherapy regimens and found the relative risk of death to be 0.85 ((0.76 to 0.96); number needed to treat for an additional beneficial outcome (NNT) = 25; absolute risk reduction = 4% (1% to 8%)). The HR for overall survival is 0.74 (0.64 to 0.89), significantly favouring the addition of postoperative platinum based chemotherapy. The HR for progression-free survival is 0.75 (0.64 to 0.89). This means that chemotherapy reduces the risk of being dead at any censorship by a quarter. Chemotherapy reduces the risk of developing the first recurrence outside the pelvis (RR = 0.79 (0.68 to 0.92), 5% absolute risk reduction; NNT = 20). The analysis of pelvic recurrence rates is underpowered but the trend suggests that chemotherapy may be less effective than radiotherapy in a direct comparison (RR = 1.28 (0.97 to 1.68)) but it may have added value when used with radiotherapy (RR = 0.48 (0.20 to 1.18)).
Authors’ conclusions
Postoperative platinum based chemotherapy is associated with a small benefit in progression-free survival and overall survival irrespective of radiotherapy treatment. It reduces the risk of developing a metastasis, could be an alternative to radiotherapy and has added value when used with radiotherapy.
Medical Subject Headings (MeSH): *Hysterectomy; Antineoplastic Combined Chemotherapy Protocols [*therapeutic use]; Chemotherapy, Adjuvant [mortality]; Endometrial Neoplasms [*drug therapy; mortality; therapy]; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans
BACKGROUND
Description of the condition
Endometrial cancer is also known as adenocarcinoma of the endometrium, uterine cancer or womb cancer. This is a malignant growth of the lining (endometrium) of the womb (uterus) classified by the World Health Organization as C54. It is distinct from sarcomas (tumours of the uterine muscle). It is the seventh commonest cancer in women worldwide with approximately 290,000 cases annually and about 75,000 related deaths (Ferlay 2004; Ferlay 2010). It affects at least 1.5% of western women in their lifetime. The chance of surviving uterine cancer depends on the stage of the cancer when it is first diagnosed.
Endometrial cancer is classified into stages according to the microscopic pattern and its ability to invade into the uterine muscle. This also determines the risk of recurrence and treatment options. The common pathological type is an endometrioid adenocarcinoma but about 10% of endometrial cancers have a serous or clear cell appearance. A small percentage are the mixed mesenchymal mullerian malignant tumour (MMMT), also known as carcinosarcoma. Mixed mesenchymal malignant tumours were initially thought to be a mixed tumour involving sarcoma and epithelial malignancy but modern pathology now recognises that they are derived from a monoclonal epithelial cell that undergoes multilineage differentiation to include sarcomatous metaplasia. All these tumours behave differently from endometrioid cancers and have a relatively poor prognosis. The stage of the cancer is based on conventional pathology examination of the resected surgical specimen. The International Federation of Gynecological Oncology (FIGO) stage system was the UICC (Union for International Cancer Control) TNM system 6 (sixth edition) until 31 December 2009. From 1 January 2010 this was superseded by TNM system 7 (seventh edition, Sobin 2009). The differences are highlighted in Table 1. A cancer that has a low risk of recurring is a well-differentiated (Grade 1 or G1) pure endometrioid tumour that has not invaded into the uterine muscle (previously classified as FIGO stage 1a) or has only invaded up to half way (previously classified as FIGO stage 1b but since 2009 classified as stage 1a). These tumours are usually cured by hysterectomy only. Intermediate risk cancer is conventionally defined as a tumour confined to the body of the uterus but is either poorly differentiated (Grade 3 or G3) and early, invading less than 50% of the way through the uterine muscle or well/moderately differentiated (grade 1/2 or G2) with invasion more than 50% of the way through the uterus (formally classified at stage 1c, now stage 1b) and when there are no other risk factors. A small number of these tumours will recur. High-risk cancers have a higher risk of recurrence and adjuvant radiotherapy reduces the risk of recurrent disease in the pelvis (Johnson 2007; Kong 2007). There is no universally accepted definition of “high risk” but the risk factors include spread beyond the body of the uterus, deep invasion through the muscle, poorly differentiated or non endometrioid type, tumour seen in the vessels of the uterus (lymphvascular invasion) and advanced age.
Table 1.
Comparison of unified UICC staging systems for endometrial cancer: TNM sixth edition (to 2009) and seventh edition (from 2010 onwards)
| Anatomic description of spread | Stage in TNM 6 | Stage in TNM 7 |
|---|---|---|
| Limited to endometrium | T1 | T1 |
|
T1a | T1a |
|
T1b | T1a |
|
T1c | T1b |
| Invades cervix | T2 | |
|
T2a | Ignore for staging purposes |
|
T2b | T2 |
| Endometrial cancer extends outside of the uterus but is confined to the true pelvis | T3 | T3 |
| Tumour invades serosa and/or adnexa and/or positive peritoneal cytology | T3a | T3a |
| Vaginal metastases | T3b | T3b |
|
T3c | T3c |
| Spread to bladder or bowel mucosa | T4a | T4a |
| Distant extrapelvic metastases | T4bM1 | T4bM1 |
It follows that the risk of micro-metastases outside the uterus after surgery determines the role of further (adjuvant) therapy. This risk can be predicted from look up tables (Lee 2006) or nomograms (Abu-Rustum 2010). Virtually all low risk tumours are cured by surgery alone and additional treatment will result in unnecessary toxicity. Some women with intermediate risk tumours may benefit from adjuvant treatment reducing the risk of relapse but the gain might be offset by the side effects. A substantial proportion of women with high risk cancers harbour micro-metastases after surgery. More women from this group will gain from adjuvant therapy. These women will have to decide if the reduction in micro-metastatic disease and the subsequent reduction in the risk of subsequent tumour progression or death is enough for them to tolerate the risks and side effects of additional treatment.
Description of the intervention
Adjuvant therapy refers to the treatment offered after surgical removal of the tumour. Adjuvant therapy can be chemotherapy, endocrine/hormonal or radiotherapy. The aim of adjuvant treatment is to destroy any cancer cells that might have spread beyond the uterus before the hysterectomy. In this analysis, we study the impact of adding chemotherapy after hysterectomy, whether this is as an additional treatment or a replacement for radiotherapy. This analysis focuses on endometrial cancer, a malignant disease of the lining of the uterus (endometrium) and this does not apply to sarcomas. The data applies to women who have had hysterectomy with curative intent and reflects a range of surgical approaches, stages and grade of disease. The benefits and harms of lymph node surgery, adjuvant endocrine (hormonal) therapy and radiotherapy are reviewed in The Cochrane Library by May 2010, Martin-Hirsch 1999 and Kong 2007 respectively.
How the intervention might work
There are several reasons why adjuvant chemotherapy has the potential to improve cure rates.
Firstly, multiple case series have shown that advanced and recurrent uterine cancer is sensitive to chemotherapy. A Cochrane meta-analysis of 11 eligible trials involving 2288 patients with advanced endometrial cancer showed that more compared to less chemotherapy significantly improves progression-free survival (PFS) (Hazard Ratio (HR) = 0.80, 95% Confidence Interval (CI) 0.71 to 0.90, P = 0.004) (Humber 2005).
The second reason for thinking it might work is that adjuvant chemotherapy improves survival after excisional surgery from other glandular cancers such as breast (Gelber 1995; van Nes 2005), colorectal (Figueredo 2008) and lung adenocarcinoma (Cheong 2007).
The third justification for adjuvant chemotherapy in high-risk but apparently completely removed endometrial cancer is the discovery that adjuvant chemotherapy increases the cure rate from ovarian cancer under the same circumstances (Winter-Roach 2009). The same might apply to uterine cancer.
A fourth reason for promoting postoperative chemotherapy for endometrial cancer is that it probably has activity in related tumours of the uterus. Both mixed mullerian malignant tumours of the uterus (Galaal 2010; Miller 2008) and uterine sarcomas (Piver 2006) respond to chemotherapy.
The fifth reason in support is that dose-dense cytotoxic chemotherapy has activity in cervical cancers and the cervix is part of the uterus (Tierney 2004) and chemotherapy improves survival rates if it is added to radiotherapy (Green 2005).
Finally, adjuvant pelvic external beam radiotherapy as a cytoxic regimen reduces the risk of pelvic recurrence by a factor of 4.6 (Johnson 2007; Kong 2007). This implies that the natural behaviour of endometrial cancer can be altered by cytotoxic treatment.
Why it is important to do this review
Postoperative chemotherapy for endometrial cancer is worthy of study because endometrial cancer is common, chemotherapy is an expensive and toxic treatment and any potential prolongation of survival needs to be balanced against the associated toxicity.
Radiotherapy may eliminate residual postoperative small volume metastatic deposits in the field that is treated. However, it will not eliminate potential recurrence from micro-metastasis outside the field of radiotherapy. Women who have an especially high risk of recurrent disease predicted from the hysterectomy laboratory analysis might have an increased survival if microscopic distant metastases beyond the field of radiotherapy were destroyed by adjuvant chemotherapy. Chemotherapy has the potential to destroy micro metastases and reduce the risk of recurrence and increase survival. This review assesses the possible effectiveness of adjuvant chemo-therapy after hysterectomy for women with endometrial cancer.
OBJECTIVES
To assess the effect of adjuvant chemotherapy on survival after hysterectomy in women with endometrial cancer and to record associated toxicities.
METHODS
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs).
Types of participants
Trial participants were women presenting with a new diagnosis of endometrial cancer treated by hysterectomy with curative intent who were considered by their treating oncologist to be fit enough to accept adjuvant chemotherapy. Pathological stage was not used to include or exclude participants but the selection will be biased against advanced stage, inoperable cases and palliative surgery.
Types of interventions
The intervention under study was adjuvant chemotherapy alone or combined with other adjuvant therapy versus any other treatment including no additional adjuvant therapy.
Types of outcome measures
Primary outcomes
Overall survival (OS) (Death rates and time to death due to any cause)
Secondary outcomes
Progression-free survival (PFS; usually defined as time to disease progression or death due to any cause)
Site of recurrences
Quality of life (QoL), measured using a scale that had been validated in a peer-reviewed publication.
- Adverse events (grade 3/4 toxicity) were grouped as haematological and gastrointestinal:
-
i)haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage)
-
ii)gastrointestinal (nausea, vomiting, diarrhoea).
-
i)
Search methods for identification of studies
We sought papers in all languages with the option of obtaining translations when necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews.
We performed an electronic search using MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) and Google (August 2010). MEDLINE was searched from 1950 to August week 1, 2010 and EMBASE from 1980 to week 32, 2010. The search strategy for MEDLINE OvidWeb, Embase and CENTRAL is presented in Appendix 1, Appendix 2 and Appendix 3 respectively. All relevant articles identified on PubMed stimulated a further search for newly published articles using the ‘related articles’ feature.
Searching other resources
Unpublished and grey literature
We searched Metaregister, Physicians Data Query, www.controlled-trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials for ongoing trials. Previously established personal communication with corresponding authors and clinical experts regularly enquired about other published or unpublished relevant trials.
Handsearching
We checked the citation list of relevant publications, abstracts of scientific meetings and list of included studies through hand searching and contacting experts in the field to identify further reports of trials. We hand searched reports of conferences from the following sources:
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist).
International Journal of Gynecological Cancer (Abstracts from the International Gynecologic Cancer Society meetings).
Annual Meeting of European Society of Medical Oncology (ESMO).
Annual Meeting of the American Society of Clinical Oncology (ASCO).
Correspondence
We contacted Bristol Myers-Squibb Company Princeton, NJ 08543 USA, originator of two standard chemotherapy agents used in many studies (carboplatin and paclitaxel) to see if they had any unpublished data in their internal records.
Language
No language restriction was applied.
Data collection and analysis
Selection of studies
We printed all titles and abstracts retrieved by electronic searching and removed duplicates. Three review authors (NJ, AB and TM) examined the remaining references independently. These three authors screened titles and abstracts of references identified from the search and eliminated articles that were obviously not relevant to the search question. Two reviewers (NJ, TM) independently assessed the eligibility of retrieved papers. We excluded those studies which did not meet the inclusion criteria and documented the reasons for exclusion. There were no disagreements although there was much debate about the need to include data from the GOG 150 trial.
Data extraction and management
Data was extracted as recommended in chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Author, year of publication and journal (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
- Endometrial cancer details
- ○ FIGO stage, using the system predating 2009
- ○ Histological cell type
- ○ Tumour grade
- ○ Presence of lymphvascular space invasion
- Intervention/control details
- ○ Adjuvant chemotherapy details
- ◇ Drug used
- ◇ Number of cycles
- ◇ Cycle length
- ◇ Dose
- ◇ Combination
- Other Adjuvant therapy details
- ◇ External beam radiotherapy details
- ◇ Brachytherapy details
- ◇ Hormone therapy details
Risk of bias in study (see below)
Duration of follow-up
Outcomes - OS, PFS, site of initial recurrence, QoL and adverse events. This included the number of participants allocated to each intervention group for each outcome of interest and the number of missing participants
We extracted data on outcomes as below:
For time to event (OS, PFS) data, we extracted the natural log of the hazard ratio (ln(HR)) and its standard error from trial reports. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. deaths, site of initial recurrence and adverse events) we extracted the number of women in each group who experienced the outcome of interest and the total number in that group at the endpoint to estimate a risk ratio (relative risk; RR) and 95% confidence interval (95% CI).
For continuous outcomes (e.g. QoL measures), we intended to extract the final value and standard deviation of the outcome of interest and the number of women assessed at the endpoint in each treatment arm at the end of follow-up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean differences (if trials measured outcomes on different scales) between treatment arms and its standard error. Only one trial reported QoL and consequently, this was not pursued.
We extracted both unadjusted and adjusted statistics, when reported.
Where possible, all data extracted were those relevant to an intention-to-treat analysis, in which participants were analysed in groups to which they were assigned.
We noted the time points at which outcomes were collected and reported.
Two review authors (NJ, AB) abstracted and analysed data independently and confirmed concordance.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using the Cochrane Collaboration’s tool and the criteria specified in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This included assessment of:
sequence generation
allocation concealment
blinding (of participants, healthcare providers and outcome assessors)
- incomplete outcome data:
- ○ We coded the satisfactory level of loss to follow-up for each outcome as:
- ◇ Yes, if fewer than 20% of patients were lost to follow-up and reasons for loss to follow-up were similar in both treatment arms
- ◇ No, if more than 20% of patients were lost to follow-up or reasons for loss to follow-up differed between treatment arms
- ◇ Unclear if loss to follow-up was not reported
selective reporting of outcomes
other possible sources of bias
The risk of bias tool was applied independently by two review authors (AB, NJ). Results are summarised in both a risk of bias graph and a risk of bias summary. Results of meta-analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
We used the HR to compare the risk of death or disease progression (event data) in the treatment group with that in the control group.
We used the RR to compare the initial treatment failure and deaths rates in each group (dichotomous outcomes).
For continuous outcomes, we intended to use the mean difference between treatment arms but no suitable data were available.
Dealing with missing data
We did not impute missing outcome data.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Assessment of reporting biases
We did not apply funnel plots corresponding to meta-analysis of the primary outcome looking for potential publication bias because we were confident that our search was exhaustive.
Data synthesis
We pooled data in meta-analyses for time-to-event data. We pooled hazard ratios using the generic inverse variance facility of RevMan 5. We calculated the relative risks for dichotomous outcomes for each study and then pooled them. We used random effects models with inverse variance weighting for all meta-analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses grouping by trials comparing chemotherapy with either radiotherapy or no additional treatment.
Sensitivity analysis
We performed sensitivity analyses by restricting the trial analysis to trials using modern conventional adjuvant chemotherapy protocols and separating trials involving mixed mesenchymal tumours from adenocarcinomas.
RESULTS
Description of studies
Results of the search
The search strategy identified 698 unique references up to August 2008. We repeated the searches in August 2010 to capture the trials that might have been published during the writing of the review. Searches of the grey literature identified additional unfinished and ongoing studies but no additional relevant studies. Recent Nordic and North Italian studies were known to the authors. Three authors independently read the titles and abstracts of the studies and excluded articles which obviously did not meet the inclusion criteria. Screening of the title and abstract produced 36 trials. Three are ongoing and listed in the table Characteristics of ongoing studies. We excluded 24 for the reasons described in table Characteristics of excluded studies. Nine randomised trials examined adjuvant chemotherapy as part of the primary treatment for uterine cancer after hysterectomy (Figure 1). They are described in the table Characteristics of included studies.
Figure 1.
The QUOROM flow diagram
Included studies
Nine trials focused on the effect of chemotherapy following the primary treatment of early endometrial cancer (EORTC 55991; GICOG; GOG 34; GOG 122; GOG 150; Hogberg-EC-9501; J GOG 2033; Kuoppala 2008; MaNGO ILIADE-3 ). The NSGO and the EORTC 55991 trials were collaborative projects and the latest published data has been combined as if it originated from a single collaborative trial.
Five trials studied the effect of adding a variety of chemotherapy regimens and agents to the treatment of women after hysterectomy and radiotherapy (EORTC 55991; GOG 34; NSGO; Kuoppala 2008; MaNGO). Four trials compared conventional adjuvant platinum based combination chemotherapy to radiotherapy (GICOG; GOG 122; GOG 150; J GOG 2033).
GOG 150 studied carcinosarcomas rather than uncomplicated adenocarcinoma. The GOG 34 and the Kuoppala 2008 used a chemotherapy regimen that would not be used in modern practice and GOG 122 and GOG 150 added abdominal radiotherapy to the pelvic treatment. Table 2 and Table 3 focus on the differences between the studies and the sensitivity analysis explores the impact of these effects on the summary statistics.
Table 2.
Main differences between the studies
| Study | Percentage with node/adnexal/distant metastases Stage 3b+ | death rate at censorship | Control arm | Comparative group | Radiotherapy dose (Gray) | Intervention arm chemotherapy | frequency of cycles |
|---|---|---|---|---|---|---|---|
| Hogberg | 1.6% | 23% | hysterectomy and pelvic radiotherapy | No additional treatment | > 44 | cisplatin 50mg/m2 + doxorubicin 50 mg/m2 or epirubicin 75 mg/m2 OR paclitaxel 175 mg/m2 + epirubicin 60 mg/m2 or doxorubicin 40 mg/m2 + carboplatin AUC 5 OR paclitaxel 175 mg/m2 + carboplatin AUC 5/6 |
4 cycles |
| J GOG 2033 | 24% | 13% | hysterectomy and pelvic radiotherapy | No additional treatment | 50 | cyclophosphamide(333mg/m2), doxorubicin (40 mg/m2), cisplatin (50mg/m2) | every 4 weeks for 3 or more courses |
| GICOG | 24% | 35% | hysterectomy and pelvic radiotherapy | No additional treatment | 45 to 50 | Cyclophosphamide 600mgm2 doxorubicin 45mgm2 and cis-platin 50mgm2 | every 28 days for five cycles |
| MaNGO | 40% | 20% | hysterectomy and pelvic radiotherapy | No additional treatment | 45 | cisplatin 50mg/m2 + doxorubicin 60 mg/m2 | every 3 weeks for 3 courses |
| GOG 34 | 42% | 36% | hysterectomy | 50 Gray pelvic radiotherapy | 50 | doxorubicin (45 mg/m2) up to a cumulative dose of 400 | 3 weekly |
| Kuoppala 2008 | 0 | 17% | hysterectomy | 56gy pelvic radiotherapy divided into two schedules with about 4 weeks break | 56 | cisplatin 50 mg/m2, epirubicin 60 mg/m2, cyclophosphamide 500mg/m2 | there chemotherapy cycles given before, during the break in radiotherapy and after radiotherapy |
| GOG 122 | 82% | 56% | hysterectomy | 30 Gray abdominal radiotherapy plus 50 Gray pelvic radiotherapy |
50 +30 to upper abdomen |
doxorubicin 60 mg/m2 plus cisplatin 50 mg/m2 | every 3 weeks for eight cycles. |
| GOG 150 | Carcinosarcoma study. 56% were stage 3 and 4 | 41% | hysterectomy | 30 Gray abdominal radiotherapy plus 50 Gray pelvic radiotherapy |
50 + 30 to upper abdomen |
Cisplatin (20 mg/m2/day×4 days) followed by a 1 hour infusion of ifosfamide (1.5g/m2/day IV×4 days) with mesna | every three weeks for three cycles |
Table 3.
Narrative summary of each trial
| Trials of adding chemotherapy versus no additional therapy after hysterectomy and radiotherapy | The Nordic NSGO trial describes 320 women after EBRT and surgery. This data are combined with an additional 63 women in the EORTC study using the same study protocol. The hazard ratio (HR) for progression-free survival was 0.64 in favour of adding chemotherapy (95% CI 0.41 - 0.99; P = 0.046). The probability of surviving 5 years if treated by chemotherapy and radiotherapy was 79% compared to 72% if radiotherapy alone was used on its own The Italian MaNGO ILIADE-3 trial describes 157 women after EBRT and surgery. Half had 3 course of cisplatin 50mg/m2 + doxorubicin 60 mg/m2 before the radiotherapy and half only had radiotherapy. The hazard ratio (HR) for progression-free survival was 0.61 in favour of adding chemotherapy (95% CI 0.33 - 1.12; P = 0.1). The probability of surviving 5 years if treated by chemotherapy and radiotherapy was 78% compared to 73% if adjuvant treatment was restricted to radiotherapy only The Finish trial describes 156 women after EBRT and surgery. Half had the radiotherapy preceded, interrupted and topped up with cisplatin, epirubicin and cyclophosphamide. The disease-specific overall five-year survival was 84.7% in women not receiving additional chemotherapy versus 82.1% in the group that did (P = 0.148). The median disease-free survival in women not receiving chemotherapy was 18 (range 9 to 36) months compared to 25 (range 12 to 49) months for women who received it (P = 0.134). The median time from surgery to recurrence was 15 (range 6 to 37) months in women not receiving chemotherapy, and 20 (range 8 to 60) months if they did (P = 0.170). Twenty-six patients died of the disease during the five-year follow-up, 11/72 from the group not given chemotherapy, and 15/84 from the group who received it. The patients succumbing from the group not receiving chemotherapy lived a median 23 (range 15 to 44) months as compared to 37 (range 13 to 50) months for those receiving it (P = 0.148) The American GOG 34 trial is over 20 years old and describes 181 women after EBRT and surgery who had doxorubicin (45 mg/m2) or no adjuvant therapy. There was no statistically significant difference in survival or progression-free interval of the two arms |
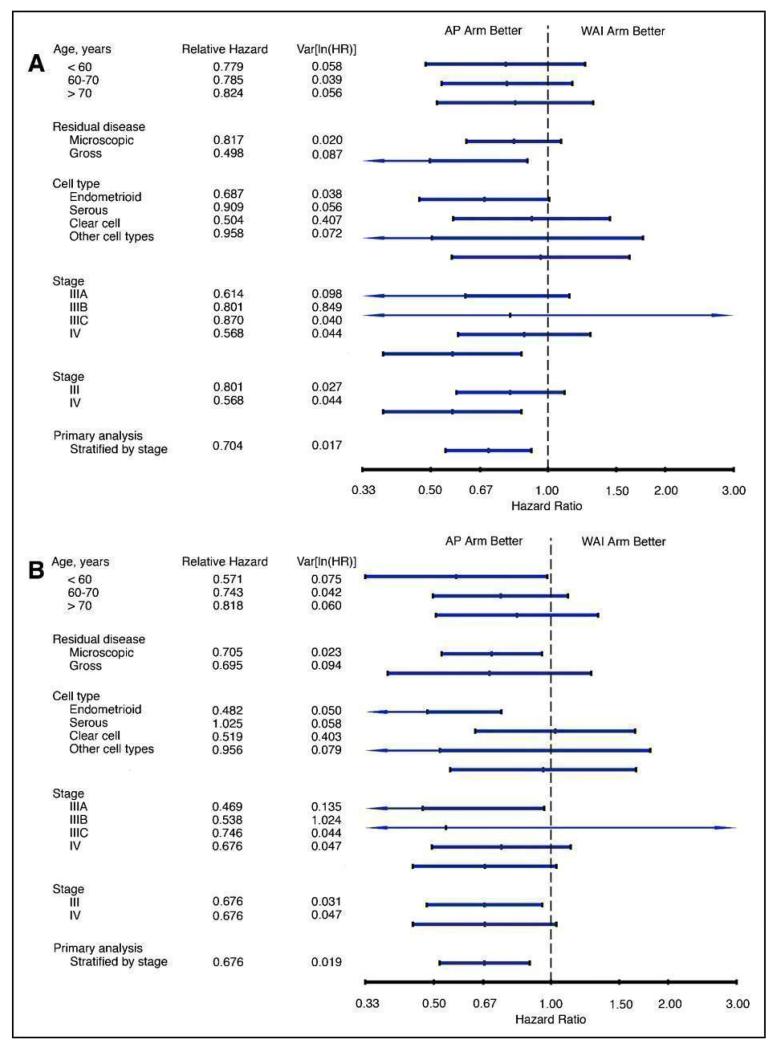
| Trials of adjuvant chemotherapy versus radiotherapy after hysterectomy | The American GOG 122 trial compared whole-abdominal irradiation with chemotherapy in 422 women after surgery. The hazard ratio presented when the data were revealed was adjusted for stage, a post hoc statistical technique that many would reject. This adjustment to the data produced a hazard ratio for progression of 0.71 favouring chemotherapy (95% CI, 0.55 to 0.91; P < .01). At 60 months, 50% of women receiving chemotherapy were predicted to be alive and disease free when adjusting for stage compared with 38% of women receiving whole-abdominal irradiation. The stage-adjusted death hazard ratio was 0.68 (95% CI, 0.52 to 0.89; P < .01) favouring doxorubicin-cisplatin chemotherapy. Again, adjusting the data for stage, at 60 months, 55% of women receiving doxorubicin-cisplatin were predicted to be alive compared with 42% of women allocated WAI The Italian GICOG trial compared adjuvant radiotherapy or chemotherapy in 345 women after surgery. The 3, 5 and 7-year overall survivals were 76%, 66% and 62% associated with chemotherapy compared to 78%, 69% and 62% after radiotherapy. The 3, 5 and 7-year progression-free survivals were, respectively, 68%, 63% and 60% and 69%, 63% and 56% The Japanese J-GOG 2033 trial describes 384 women equally divided to adjuvant CAP chemotherapy or pelvic radiotherapy after surgery. This trial did not report any statistically significant differences in overall progression-free survival (PFS) and overall survival (OS). The five-year progression-free survival rates associated with chemotherapy or radiotherapy was 81.8% and 83.5% respectively, while the five-year overall survival rates were 86.7% and 85.3% respectively. These rates were not significantly different if the analysis was restricted to a low- to intermediate-risk group (defined as stage IC patients under 70 years old with G1/2 endometrioid adenocarcinoma). However, chemotherapy was associated with a significantly higher progression-free survival rate (83.8% versus 66.2%, and higher overall survival rate (89.7% versus 73.6%) in a subgroup analysis of the 120 women with a high- to intermediate-risk cancer defined as invasion to the outer half of the myometrium in women over 70 years of age or G3 endometrioid adenocarcinoma or tumour involving any part of the cervix or positive cytology The American GOG 150 trial describes 206 women with a primary mixed mullerian tumour after surgery treated either by whole-abdominal irradiation or up to three cycles of cisplatin, ifosfamide and mesna. The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). Adjusting for stage and age, the recurrence rate was 21% lower for CIM patients than for WAI patients, (relative hazard (RH) = 0.789, 95% confidence interval (CI): (0.530 to 1.176), P = 0.245, 2-tail test). The estimated death rate was 29% lower among the CIM group (RH = 0.712, 95% CI: 0.484 to 1.048) |
Trials of adjuvant chemotherapy versus no additional therapy after surgery and radiotherapy
NSGO & EORTC
The collaborative Nordic (NSGO) and European trials (EORTC 55991) ran in parallel using the same protocol. The Nordic Gynaecology Oncology Group and the European Organisation for the Research and Treatment of Cancer studied the effect of randomly allocating a platinum based chemotherapy regimen against no additional treatment. The trial participants all had a hysterectomy and radiotherapy for endometrial cancer. Initially, the trial was exclusively studying cancers confined to the corpus but a later amendment allowed stage 2 and 3 to be included. The exact choice of chemotherapy regimen varied over the recruitment period of the trial but all were derived from standard active published regimens used in trials of chemotherapy in advanced disease. Before August 2004, chemotherapy consisted of four courses of cisplatin 50 mg/ m2 + doxorubicin 50 mg/m2 or epirubicin 75 mg/m2. Thereafter several chemotherapy regimens were allowed, of which cisplatin 50 mg/m2 + doxorubicin 50 mg/m2, paclitaxel 175 mg/m2 + epirubicin 60 mg/m2 + carboplatin AUC 5, and paclitaxel 175 mg/ m2 + carboplatin AUC 5-6 were used. The pivotal presentation at the 2007 ASCO meeting describes 382 women. Data are now available from 383 women, 320 from NSGO and 63 from the EORTC. The EORTC have not yet published independent data but early published results are available from ESMO and ASCO presentations and the collaborative data on 534 evaluable women including data from MaNGO was published in 2010 (NSGO, MaNGO and EORTC). Risk factors were well balanced between the randomisation arms. The primary outcome endpoint was progression-free survival defined as the time from randomisation to progression of endometrial cancer or death from all causes. Secondary end-points included overall survival.
MaNGO
The Mario Negri Gynecologic Oncology group (MaNGO) is a collaborative Italian group funded by Italian Agency for Drugs. ILIADE3 is the third important endometrial cancer trial by this group and compared the survival of 157 women who were randomly allocated no additional treatment or doxorubicin 60mg/m2 + cisplatin 50 mg/m2 every 3 weeks for three cycles after surgery but before radiotherapy. It is similar to the Nordic (NSGO) and European trial (EORTC 55991) but recruited a larger proportion of more advanced stage cases. It was exclusively concerned with endometroid adenocarcinoma pathology. The treatment arms were well balanced regarding prognostic factors. The primary outcome endpoint was progression-free survival defined as the time from randomisation to progression of endometrial cancer or death from all causes. Secondary end-points included overall survival.
Kuoppala 2008
Kuoppala 2008 was a multi institution national randomised trial from Finland examining the effect of adding platinum-based chemotherapy or not in 156 women with high-risk endometrial cancer after standard surgery and radiotherapy. The objective was to measure the overall and disease-free survival and recurrence rates in women with intermediate or high-risk endometrial cancer. The women had Stage Ia or 1b Grade 3 (n = 28), or Stage pT1c-pT3a grade 1-3 (n = 128) (using the UICC TNM 6th edition staging prior to 2009-Table 1). They were randomised postoperatively to receive radiotherapy (56 Gray) only (“Group A”, n = 72) or radiotherapy in a sandwich regimen combined with three courses of cisplatin (50 mg/m2), epirubicin (60 mg/m2) and cyclophosphamide (500 mg/m2) (“Group B”, n = 84). The radiotherapy was given in two courses of 28 Gray each, separated by a pause of three weeks. The chemotherapy was given in three courses. The first was given after surgery and before radiotherapy. The second was given in the pause in radiotherapy and the final course was given within 2 weeks of completing the final radiation treatment. The authors reported disease-specific overall five-year survival and median disease-free survival and time from surgery to recurrence.
GOG 34
The Gynecologic Oncology Group protocol 34 examined the addition of doxorubicin (Adriamycin®) versus no additional treatment after surgery plus external beam radiation therapy. This randomised, prospective trial studied women with clinical stage pT1 or pT2 (occult) endometrial cancer who, after surgical-pathologic evaluation, had one or more risk factors for recurrence: greater than 50% myometrial invasion, pelvic or aortic node metastasis, cervical involvement, or adnexal metastases. All women without aortic node metastasis received 50 Gray to the whole pelvis at 1.6 to 1.8 Gray per day. If aortic node metastasis was documented, additional para-aortic field radiation extending superiorly to the T11-T12 interspace was offered. The aortic target dose was 45 Gray at 1.5 Gray per day. After completion of radiation therapy, women were randomised to receive doxorubicin bolus therapy (60 mg/m2 starting dose) to a maximum cumulative dose of 500 mg/ m2. Ninety-two women were entered into the doxorubicin treatment arm, and 89 patients entered the arm with no additional treatment.
The trial reported overall and progression-free survival, as well as the five-year survival rates for women with deep myometrial invasion, cervical involvement, pelvic node metastases and patients with aortic node metastases.
Trials of adjuvant chemotherapy versus radiotherapy after surgery
GOG 122
The Gynecologic Oncology Group protocol 122 compared whole-abdominal irradiation (WAI) with chemotherapy in 422 women with UICC 6th edition stage pT3 or pT4a endometrial carcinoma having a maximum of 2 cm of postoperative residual disease after surgery. Three hundred and ninety-six women were assessable. A total of 202 were randomly allocated to receive WAI, and 194 were allocated to receive chemotherapy. Radiotherapy involved 30 Gray at 1.5 Gray per day to the whole abdomen with a 15 Gray boost to the pelvis. Chemotherapy consisted of doxorubicin 60 mg/m2 and cisplatin 50 mg/m2 every three weeks for seven cycles, followed by one additional cycle of cisplatin designed to restrict the possible cumulative cardiac toxicity of doxorubicin. Most patient and tumour characteristics were well balanced. The median age was 63 years; 50% had endometrioid tumours. Median follow-up time was 74 months. However, GOG 122 was not a purely adjuvant trial because the recruitment criteria included residual tumour up to 2 cm and the radiotherapy dose was insufficient to treat residual tumour of this size. In contrast, the chemotherapy was extensive and toxic (8 cycles). The trial reported a crude hazard ratio and a hazard ratio adjusted for stage for overall survival and disease-free survival.
GICOG
This Italian trial randomly assigned adjuvant radiotherapy or chemotherapy after hysterectomy to 345 women with high-risk endometrial carcinoma (UICC 6th edition stage pT1cG3, pT2G3 with myometrial invasion greater than 50%, and pT3). Chemo-therapy involved 5 cycles of cisplatin (50 mg/m2), doxorubicin (45 mg/m2) and cyclophosphamide (600 mg/m2) every 28 days. Radiotherapy involved 50 Gray external beam to the pelvis on a 5 days week schedule in 45 fractions. Median follow-up at the time of reporting was 8.0 years. The primary end points were overall and progression-free survival. The authors reported a hazard ratio advantage for overall and progression-free survival, as well as overall and progression-free survival after 3, 5 and 7 years.
J GOG 2033
This Japanese multi-centre randomised trial compared adjuvant pelvic radiation therapy with a combination of cyclophosphamide, doxorubicin (Adriamycin-®), cisplatin (CAP) chemotherapy. 103 institutions randomised 385 women with UICC 6th edition stage pT1c-pT3c and > 50% myometrial invasion (stage pT2 or pT3 with < 50% invasion were ineligible). Stage distribution was pT1c 61%, pT2 14%, pT3a 13%, pT3c12%. Women were randomly allocated pelvic radiotherapy (n = 193) or CAP chemotherapy (n = 192). External beam radiotherapy delivered 45 to 50 Gray via anterior-posterior opposed beams. The CAP chemotherapy regimen involved cyclophosphamide (333 mg/m2), doxorubicin (40 mg/m2) and cisplatin (50 mg/m2) every four weeks for three or more courses. The trial reported overall and progression-free survival, as well as the five-year overall and progression-free survival rates. These rates were further analysed in low- to intermediate-risk groups (defined as stage pTIc in women under 70 years old with grade1 or 2 endometrioid adenocarcinoma) and high- to intermediate-risk cancer (defined as invasion of tumour to the outer half of the myometrium in women over 70 years of age) or G3 endometrioid adenocarcinoma or tumour involving any part of the cervix or positive cytology (TNM 6th edition pT1c and > 70years, pT1cG3, pT2, pT3a).
GOG 150
The GOG 150 trial focused on women with mixed mullerian mesenchymal malignant tumours (also called carcinosarcoma). Many experts believe these tumours originate from endometrial cancer cell lines but this view was not universally held in the past. Consequently, the meta-analysis studying the impact of chemotherapy for women with endometrial cancer is presented including these data for those who believe this tumour is a cancer and it is presented excluding this data for those readers who prefer to consider the tumour as a separate entity. The trial compared chemotherapy (n = 101) with whole body radiation (n = 105) in women presenting for the first time with any stage of primary mixed mullerian mesenchymal malignant tumour of the uterus or cervix without demonstrable parenchymal hepatic involvement or extra-abdominal distant disease. Surgical staging revealed that 44% of women had disease beyond the uterus and cervix (stage III-IV). The chemotherapy comprised intravenous cisplatin (20 mg/m2/day × 4 days) followed by a one hour IV administration of Ifosfamide (1.5 g/m2/day IV × 4 days) with mesna (120 mg/m2 IV bolus over 15 minutes on day one, followed by 1.5 g/m2/day IV continuous infusion × 4 days beginning with day one) every three weeks for three cycles. Radiotherapy initially involved hyperfractionation doses but this was changed to daily doses due to poor accrual. The abdomen received 30 Gray, the pelvis received approximately 50 Gray.
The authors reported the estimated overall survival and the crude probability of disease recurring within 5 years and the adjusted rate accounting for stage and age.
Excluded studies
We excluded 12 studies because they studied women with recurrent or advanced or incurable primary disease. Eleven of these are analysed in detail by Tierney 2004. An additional study byBrunetto 2000 studied the addition of ifosfamide alone or in combination with cisplatin in the treatment of advanced, persistent or recurrent carcinosarcoma of the uterus. Several other papers were excluded from the analysis because the primary pathology included pure sarcoma. Omura studied all sarcomas and Samuels 2004 specifically focused on leiomyosarcomas and liposarcomas. Three studies compared different regimens (Chauvergne 2008; Deng 2000; Fujimura 2000). Chauvergne 2008 compared different chemotherapy agents, had no other comparative group and did not use randomisation to allocate the treatments. Fujimura 2000 compared different chemotherapy regimens rather than evaluating chemotherapy against other treatment. Deng 2000 examined the morphological changes induced in endometrial cancer by radio-therapy and chemotherapy but the outcomes were not recorded. Two other trials were closed early and no data are available. Both were known to the authors but not found by electronic searching. GOG 156 compared pelvic radiotherapy with doxorubicin and cisplatin after hysterectomy. Women had endometrial cancer ranging from minimal invasion of the myometrium (formally called stage 1b) to invasion of the cervical stroma (formally called stage 2b). GOG 194 was a randomised study with adjuvant postoperative irradiation with or without the addition of Cisplatin/ Taxol chemotherapy. The primary outcome was relapse-free survival from endometrial cancer. Neither GOG 156 nor GOG 194 recruited adequately and the trials were closed due to poor accrual. For further details of all the excluded studies see the table Characteristics of excluded studies.
Ongoing studies
GOG 249, GOG 258 and PORTEC 3 are ongoing trials and details are described in Characteristics of ongoing studies.
Risk of bias in included studies
Full details are available in the risk of bias table within the table Characteristics of included studies.
Allocation
All trials used a specialist independent data centre and randomisation appears secure.
Blinding
Data analysis for all trials was performed in a recognised data centre and the risk of statistical manipulation from awareness of the treatment allocation is negligible.
Incomplete outcome data
All trials reported the denominators and gave explanations when the data set was incomplete. All trials had a high data acquisition rate.
Selective reporting
Details of selective reporting from GOG 122 and J GOG 2033 are given in the table describing the Risk of bias in included studies.
Effects of interventions
Overall effect of chemotherapy on survival rates
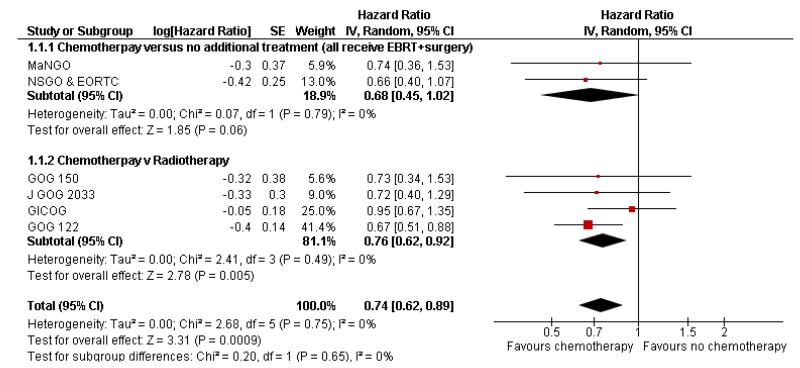
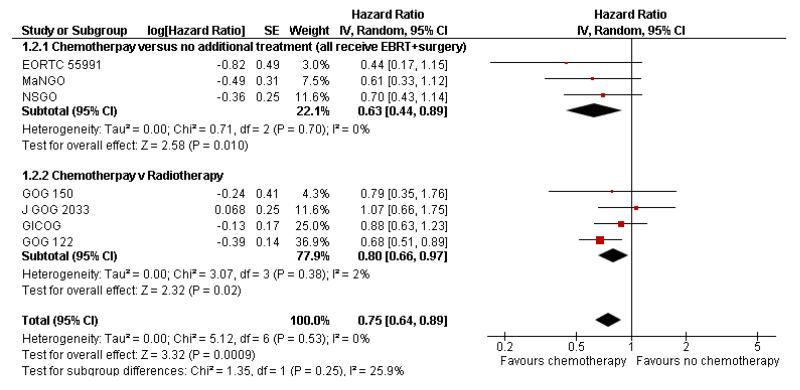
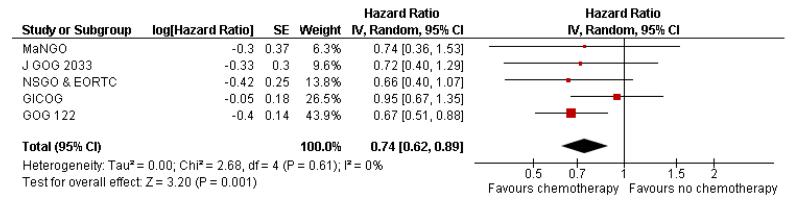
Chemotherapy is associated with an improved survival and a longer disease free survival. The hazard ratio compares survival curves and represents the risk of death over time if chemotherapy is added to the treatment regimen, either as an addition to surgery and radiotherapy or instead of radiotherapy. The hazard ratio (HR) of death from any cause is 0.74 (0.62 to 0.89). This represents a significant overall survival advantage for women in these trials (Analysis 1.1, Figure 2). There is a similar progression-free survival advantage (HR = 0.75 (0.62 to 0.89)) (Analysis 1.2, Figure 3). The probability that this is a chance finding is less than one in a thousand (P = 0.0009). The data are not affected by any significant variability or distortion due to heterogeneity (I2 = 0%).
Figure 2.
Forest plot from all trials of the hazard ratio for death from any cause (representing overall survival).
Figure 3.
Forest plot from all the trials of the hazard ratios for death or recurrence (representing progression-free survival).
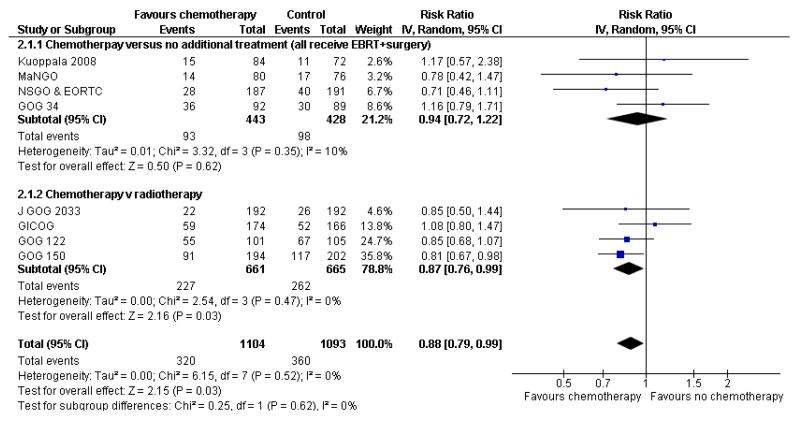
Nine trials allow a comparison of death rates for chemotherapy versus any other arm. Survival data 5 years after randomisation from 2197 women gave a relative risk of 0.88 (95% CI; 0.79 to 0.99) (Figure 4, Analysis 2.1). This indiscriminate analysis represents an odds ratio of 83% and a risk difference of 3% (95% CI;0.01 to 0.07) This represents an absolute change in risk attributable to chemotherapy for one women in every 33 who are treated; NNT = 33; Analysis 2.2.
Figure 4.
Indiscriminate forest plot for overall survival (risk of death 5 years after randomisation) from all trials of chemotherapy versus any other arm.
Senstivity analysis
Senstivity analysis; separating the trials into comparisons of chemotherapy versus radiotherapy and chemotherapy versus no additional treatment
Comparing chemotherapy or radiotherapy
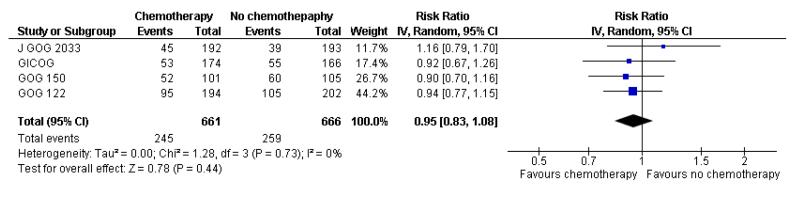
Four trials (GICOG; GOG 122; GOG 150; J GOG 2033) compared chemotherapy directly with radiotherapy after surgery. The pooled data meta-analyses show a statistically significant improvement in survival rates (risk ratio (95% CI) = 0.76. (0.62 to 0.92)) when chemotherapy is compared with radiotherapy (Analysis 3.1). There is a similar advantage in progression-free survival (HR = 0.80; 0.66 to 0.97). Data from four trials involving 1326 women show a risk ratio (relative risk of death at five years) of 0.87 (0.76 to 0.99), Analysis 3.3. The absolute risk reduction in death attributable to the chemotherapy is 4%. Twentyfive women would need to be treated to treat to save one life. The hazard ratio remains the same (0.76 (0.61 to 0.96); n = 1120) if the analysis excludes trials focusing on mixed tumours with potentially different biology (GOG 150). However, GOG 122 was not a pure adjuvant trial because it included residual tumour up to 2cm and the radio-therapy may not have been adequate for this volume of disease. The statistical significance is lost if this trial is omitted from this analysis (overall survival advantage HR= 0.86 [0.65, 1.14]). Nevertheless, the percentage of the variability in effect estimates due to heterogeneity rather than chance is negligible (I2 = 0%).
Comparing the addition of chemotherapy versus no treatment after surgery and radiotherapy
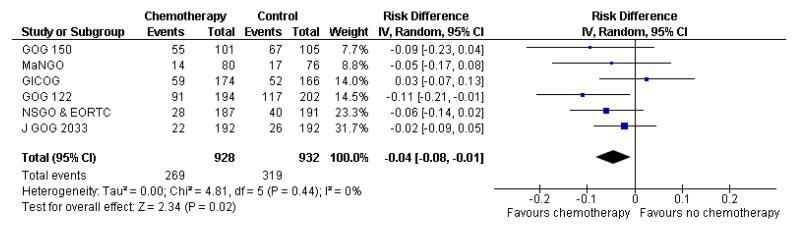
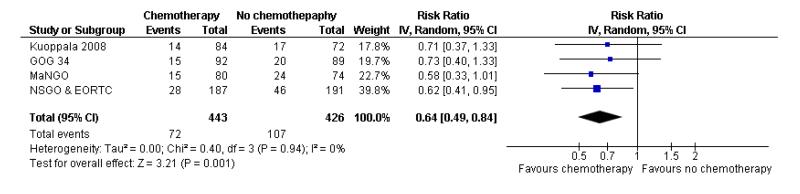
We assessed the impact on survival from adding chemotherapy versus no additional treatment by pooling GOG 34, Kuoppala 2008, MaNGO and NSGO & EORTC (Hogberg). In these trials, all women had surgery plus pelvic radiotherapy. This analysis reveals a similar effect but the analysis lacks power due to the smaller sample size (n = 871) and there is significant variation in the summary findings implying that the trials are heterogenous (I2 = 32%). This means that the trials may not be studying the same intervention and it may be mathematically inappropriate to combine them. Kuoppala 2008 and GOG 34 studied a chemo-therapy regimen that would not be used today whilst MaNGO,EORTC 55991 and NSGO studied conventional platinum based regimens. Therefore, a case can be made for separating these trials. If NSGO, MaNGO and EORTC only are analysed, the overall survival and progression-free advantage HR from receiving chemotherapy is 0.68 (0.45 to 1.02; Analysis 4.1) and 0.63 (0.44 to 0.89; Analysis 4.2) respectively. The risk of death at five years is may be reduced by chemotherapy RR = 0.74 (0.51 to 1.06), risk difference 6% (-1% to 12%). However, inclusion of the Kuoppala 2008 and GOG 34 data (Analysis 4.3) caused the data to be heterogeneous (I2 = 10%) and the analysis cannot detect any difference in the risk of death at five years (RR = 0.94 (0.72 to 1.22)).
Senstivity analysis; excluding mixed mesenchymal tumours
Mixed mesenchymal tumours may have different biology but excluding the data from these tumours (GOG 150) does not significantly affect the summary statistics. The hazard ratio for overall survival and progression-free survival is the same irrespective of whether the data from mixed mesenchymal tumours are included or excluded (Analysis 5.1, Figure 5). The risk ratio also remains at 0.89 (0.78 to 1.02) if women with mixed tumours (carcinosarcomas) are excluded (Analysis 5.2; n = 1991) and 0.85 (0.74, 0.98) if women with mixed tumours and trials of unconventional chemotherapy are excluded. Again, there is no significant variability in estimates due to heterogeneity (I2 = 0%).
Figure 5.
Forest plot of overall survival excluding mixed tumours (carcinosarcomas)
Senstivity analysis; using adjusted or unadjusted hazard ratios
The GOG 122 study reported a hazard ratio adjusted for stage. Other trials report the unadjusted statistic. Sensitivity analysis shows that this is unimportant as the combined hazard ratio for both overall survival and progression-free interval is similar whether the adjusted or the unadjusted hazard ratio is used. The unadjusted hazard ratio recalculated from the survival curves of GOG 122 yields a summary statistic (combined Hazard ratio for overall survival 0.77 (0.65 to 0.92) compared to 0.74 (0.62 to 0.89) if the adjusted figure is used.
Senstivity analysis; excluding unconventional chemotherapy regimen such as doxorubicin monotherapy or split course regimens
Hogberg 2008’s primary hypothesis was that chemotherapy has limited value unless it is given with radical adjuvant intent. This would exclude Kuoppala 2008 because it is a trial sandwiching chemotherapy in between radiotherapy and could be considered to be a trial of radiation sensitiser, rather than a trial of adjuvant chemotherapy. Similarly, GOG 34 only used single agent doxorubicin and no platinum agent. Studies of chemotherapy in advanced disease suggest a significantly higher activity with multi-agent regimens. Analysis 2.3 excludes these two trials and shows that the relative risk of death during the trial period is 0.85 (0.76 to 0.96; n = 1860). This is consistent with a significant survival advantage to chemotherapy (Figure 6). This equates to an absolute risk reduction of 4% (95% CI; 1% to 8%). If these data were replicated in practice, 25 women would need to receive modern chemotherapy for one to avoid death (Analysis 2.4). There is minimal variability in effect estimates due to heterogeneity (I2 = 0%) despite the fact that this combines data from trials in which chemotherapy is added to surgery or is an alternative to radiotherapy.
Figure 6.
Forest plot showing overall survival (risk of death) restricted to trials of high dose chemotherapy.
Restricting the analysis to only include trials of modern platinum based chemotherapy regimens is associated with funnel plots that demonstrate greater homogeneity. This means that there is minimal risk of contamination by heterogeneity.
Patterns of Initial Treatment Failure; risk of pelvic recurrence
Risk of extra-pelvic recurrence
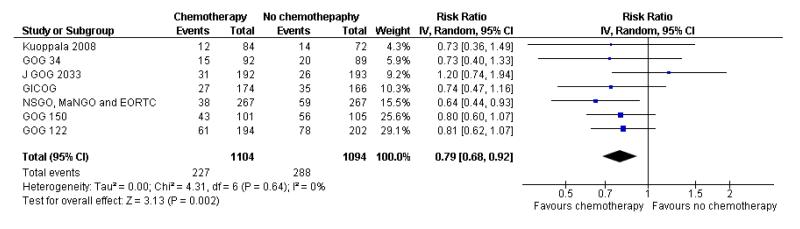
All included trials reported the risk of developing the first recurrence outside the pelvis. Data are available on 2198 women (Analysis 6.1). The risk ratio is 0.79 (95% CI; 0.68 to 0.92) implying that chemotherapy reduces the chance that the first site of recurrence will be outside the pelvis (Figure 7). This translates to a 22% relative risk reduction, 5% absolute risk reduction (Analysis 6.2). The number needed to treat to prevent one recurrence is 20. The variability in effect estimates attributable to heterogeneity rather than chance is negligible (I2 = 0%) and the result is similar if the GOG 150 carcinosarcoma trial is excluded (Analysis 6.3).
Figure 7.
Forest plot comparing the risk of developing the first metastasis outside the pelvis
Risk of pelvic recurrence; chemotherapy verus no additional treatment after hysterectomy and radiotherapy
GOG 34, Kuoppala 2008 and NSGO, MaNGO and EORTC 55991 examine the impact of adding chemotherapy to surgery and radiotherapy. The GOG 34 does not report data on the risk of pelvic recurrence but we know that there is no significant difference in rates. That leaves data from 690 women to analyse. Chemotherapy appears to show a trend to lower rates of recurrent disease in the pelvis but this calculation is underpowered due to the small sample size. The difference is not statistically significant (risk ratio (95% CI) = 0.48 (0.20 to 1.18) (Analysis 6.4). However, the only available data from NSGO, MaNGO and EORTC 55991 have been summated and this crude pooling of data does not lend itself to accurate meta-analysis risk ratio calculations.
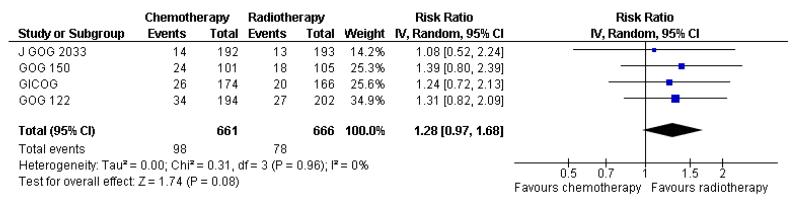
Risk of pelvic recurrence; chemotherapy versus radiotherapy
The Italian GICOG, American GOG 122, Japanese J GOG 2033 and the GOG 150 carcinosarcoma trial give data on the rate of pelvic relapse comparing radiotherapy to chemotherapy. These four trials report the first site of recurrence for 1327 women. There is a trend towards chemotherapy being associated with more recurrent disease first becoming apparent in the pelvis compared to radiotherapy (Figure 8). This is not statistically significant; risk ratio (95% CI) = 1.28 (0.97 to 1.68) (Analysis 6.5). The risk ratio = 1.24 (0.90 to 1.70) if the GOG 150 carcinosarcoma trial is excluded.
Figure 8.
Forest plot comparing the risk of an recurrence initially seen in the pelvis with chemotherapy or radiotherapy.
Separating the site of recurrence shows that both radiotherapy and chemotherapy have activity. One reason why there may be an increase in the risk of metastasis presenting initially in one site rather than another may not be the failure to control disease. A reduction in the incidence at one site might mean women will live long enough to develop a recurrence elsewhere. What matters to women is the risk of a recurrence at any site. The location of that site then becomes important because salvage therapy depends on the location of the recurrence and the prior treatment.
Risk of any recurrence at any site
Chemotherapy reduces the risk of developing an initial recurrence outside the pelvis (Analysis 6.1). It might have added value and reduce the risk of pelvic recurrence when used with radiotherapy (Analysis 6.4) but the risk of pelvic recurrence might increase if radiotherapy is omitted (Analysis 6.5). The impact of this is shown by studying the risk of any recurrence at any site.
There is no detectable difference between chemotherapy and radiotherapy in the overall risk of a metastasis (RR = 0.95 (0.83 to 1.08)). This is based on four trials studying 1327 women and is illustrated in Figure 9; Analysis 6.7. However, chemotherapy significantly reduces the risk of a recurrence at any site (risk ratio (95% CI) = 0.64 (0.41 to 0.84) compared to no additional treatment (after surgery and radiotherapy). The combined data from Hogberg means that Analysis 6.6 has 4 data sets. In other words, a woman will have an additional reduction in the risk of a recurrence even if she is having radiotherapy (Analysis 6.6, Figure 10).
Figure 9.
Forest plot examining the risk of a metastasis at any site with chemotherapy compared to radiotherapy.
Figure 10.
Forest plot comparing the risk of a recurrence after surgery and radiotherapy at any site with chemotherapy compared to no adjuvant treatment ).
Subgroup analysis
The impact of chemotherapy on serous papillary or clear cell carcinoma is studied in Analysis 7.1 and Analysis 7.2. The effect of chemotherapy in this subgroup seems similar to the impact for all cancers. However, the small numbers in this subgroup mean that the analysis lacks power and the beneficial effect cannot be confirmed (Overall survival HR = 0.98 (95% CI; 0.68 to 1.40) and progression-free survival HR = 0.84 (95% CI; 0.57 to 1.23))
Toxicity
GOG 122 compared whole-abdominal irradiation with doxorubicin-cisplatin chemotherapy in 422 women. It was the only study to report quality of life (Bruner 2007). In this intention-to-treat analysis on 388 eligible patients, no statistical differences in Assessment of Cancer Therapy-General (FACT-G) scores were identified at any assessment time between the two groups. In terms of fatigue, the only difference between the groups was shown at the end of treatment with women having whole abdominal radio-therapy reporting significantly higher fatigue compared to women on chemotherapy (P < .01). Fatigue and elimination problems were acutely worse for women having radiotherapy but the score levels off at 6 months to close to pre-treatment levels. However, marked peripheral neuropathy was sustained for at least 6 months for women receiving chemotherapy.
Each trial reports some toxicity data and this is listed in the table of Characteristics of included studies but this is insufficient to estimate the gains in the form of quality-adjusted benefit. The relationship between chemotherapy treatment toxicity and global quality of life effects is not clear (Butler 2004; Luoma 2002) and this has significant implications when deciding the overall gain to patients where the Quality Adjusted Life year is the preferred measure of incremental benefit.
There is insufficient agreement between the reporting of trials to make any meaningful comments about serious adverse events. However, rare serious toxicities associated with chemotherapy are known for many regimens and the risk for each patient needs to be individualised. Data from trials is not the most appropriate tool to assess rare events but the trial data are abstracted in the toxicity section of the Characteristics of included studies.
DISCUSSION
Summary of main results
Adjuvant chemotherapy improves overall survival, progression-free survival and reduces the risk of recurrent metastases. It seems to have a greater effect on survival than radiotherapy and has added value when used with radiotherapy.
Radiotherapy is an effective cytotoxic treatment but its benefits are limited to reducing pelvic relapse. In contrast, chemotherapy has a systemic effect and this is probably why it is associated with the additional reduction in the rate of distant extrapelvic metastases. The proportion of women having micro-metastatic disease is greatest in high-risk disease, and the effects of adjuvant therapy are easiest to show in this group. For example, the magnitude of effect was small in the J GOG 2033 trial. In the early ASCO presentations of this trial, 208 women had low or intermediate risk disease with an overall relapse rate of 10%. Adjuvant treatment in this subgroup had little demonstrable effect but the 67 women with high-risk disease and a recurrence rate of 25% gained a substantial benefit from adjuvant chemotherapy. The full report (J GOG 2033) has slightly different numbers and this is explored in the table of bias. In contrast to the trials studying women with low risk pathology, the magnitude of the effect in GOG 122 was large and this trial had a survival rate below 50%.
The beneficial effect of chemotherapy seems to be limited to postoperative platinum based sequential regimens. Unconventional and non standard regimens such as single agent doxorubicin (GOG 34) or chemotherapy sandwiched around and interrupting radiotherapy had little impact on survival (Kuoppala 2008). It seems that chemotherapy is most effective when using conventional platinum-based regimens.
As global QoL comparisons were only included in one randomised trial, it is difficult to quantify the trade-off between toxicity and benefit from chemotherapy. We know that women with certain stages of ovarian cancer, bowel cancer and breast cancer will risk the toxicity of adjuvant chemotherapy for absolute benefits in the range of 5% to 10%. This suggests that some women with high-risk endometrial cancer might accept the toxicity of platinum based multi-agent chemotherapy for the likely but small survival gain identified by this meta-analysis.
Overall completeness and applicability of evidence
Contact with trials groups and registries, as well as with reviewers has made publication bias unlikely. We feel that it is very unlikely that the study coordinators or our reviewers have missed a large negative trial with data of sufficient power to reverse our conclusion. Therefore, the evidence in this review is as complete as it can be.
The applicability of this work to practice is more controversial. All this review can say is that adjuvant chemotherapy has activity, reduces the risk of distant metastases and improves survival rates. This finding is clearly relevant to policy makers who should make an economic assessment, evaluate resources and consider making adjuvant chemotherapy available to selected high-risk groups. However, the applicability of the findings of this review to clinical practice is much more difficult to assess. This review does not help the clinician choose who to treat, when to treat or what treatment to use. The only implication of these data is that clinicians should consider platinum based chemotherapy and radiotherapy, assess the prior risk of distant and local metastases, titrate the obvious harms and risks from a chosen chemotherapy regimen and support the patient as she makes a difficult decision about her adjuvant therapy options.
Quality of the evidence
This is the first study to be able to include more than 2000 women with endometrial cancer who were randomised between adjuvant treatments with chemotherapy or not. The magnitude of the effect of adjuvant chemotherapy is small, with an absolute difference of only 3% in the overall study. Individual trials were underpowered to confirm this effect. This study also informs prospective trial designs and data monitoring committees of the required sample size to achieve adequate statistical power. Small trials with low event rates will be be underpowered if they are examining a survival advantage.
The strength of this study is that it allows all the data to be analysed together, to produce one result. Many of the individual contributing trials lacked statistical power and performed subgroup analyses and potentially unjustified data adjustments to make claims of benefit (GOG 122; J GOG 2033). The claims from individual trials that there was an overall survival benefit with chemotherapy was difficult to accept when there was no improvement in progression-free survival to suggest that more active cancer treatment was the cause. This study overcomes these issues.
While many groups have argued that histological subtypes of endometrial cancers may have different natural histories, other publications show the opposite. In other sub studies based on reanalysis by different groups, stage or pathology type has been used to suggest an effect. This has been used to justify secondary trial data analysis with conclusions unsupported by the total evidence. In contrast, this study has permitted all the different histological types of endometrial cancer to contribute to the data as intended as studies recruited in a primary analysis with a clear conclusion. The absence of heterogeneity from this broader inclusion strategy suggests the results of the study have general applicability.
A weakness of meta-analysis is that sources of bias are not controlled by the method. A good meta-analysis of badly designed studies will still result in bad statistics. Assessment of the risk of bias that may affect the cumulative evidence in this study is reassuring. The ability to calculate the effect with the unadjusted total data from 8 data sets with more than 2000 trial participants overcomes the well-recognised selective reporting and publication within the individual studies themselves. Further reassurance about data bias is that the search strategy produced only trials monitored by experienced national or supra-national trials groups. Each group has a long record of publication; practises external review and has independent data monitoring. Inhomogeneity in meta-analysis is a frequent driver of bias. The low I2 seen in the calculations suggests that this effect will not bias the result. Further strength comes from sensitivity analysis. While individual studies have made sub-studies of the competing effects of radiotherapy and chemotherapy, the sensitivity analysis of this meta-analysis shows the treatment effect is present irrespective of whether chemotherapy was compared with radiotherapy or with no adjuvant treatment.
The weakness of the study is that only one of eight contributing studies reported Quality of Life scores in each arm. This means that while we may be certain that there is a survival benefit from chemotherapy, we are uncertain whether the survival gain is outweighed by toxicity. This means that economic analyses of the cost effectiveness of adjuvant chemotherapy will have to be based on “added life year” gains (ALY) rather than “quality-adjusted life year” gains (QALY).
Potential biases in the review process
We performed a comprehensive search. Three reviewers (NJ, TM and AB) independently sifted all studies and extracted data. We restricted the included studies to RCTs as they provide the strongest level of evidence available. This will minimise bias. The greatest threat to the validity of this type of review is the possibility of publication bias i.e. studies that failed to show a difference may not have been published. The authors are experts in the field and we believe that it is very unlikely that we have overlooked a significant contribution to this topic. The main bias associated with reviews of this type are the heterogeneity of the case mix and treatment regimens. Statistical tests for heterogeneity in this review show that this is unlikely to bias the conclusion although it is a major confounding variable when assessing the magnitude of the effects of chemotherapy.
One potential bias is acceptance of the Hazard Ratio quoted in the GOG 122. These data were adjusted by the trial authors to account for perceived imbalances in treatment allocation state for stage. The validity of this and the interpretation of risk in this analysis can been criticised. There are good statistical reasons why the meta-analsyis should use the unadjusted HR. The estimated unadjusted HR from the survival curves for overall survival in GOG 122 is estimated to be 0.74 (0.57 to 0.96). This is less impressive than the headline figure quoted by the trial authors (0.67 (0.51 to 0.88)). Nevertheless, even if the unadjusted and less dramatic headline figure is used, the calculated meta-analysis HR using the inverse variance random effects model remains similar. Sensitivity analysis also demonstrates that the analysis is robust even if the GOG 150 data are excluded on the basis that this is a trial of mixed tumours. Futhermore, the sensitivity analysis shows that the data are robust if different meta-analysis tools are used.
One other issue is the number of subgroup analyses. This review has deliberately chosen to publish the calculated difference in the primary outcome using several different tests. This is not normal good statistical practice. Statistics is an art and P value can change according to the chosen method of analysis. Therefore, multiple calculations risk producing a result that is statistically significant by chance alone. The traditional concept that a P value must be 5% or less now has less credibility than in the past. Doctors now appreciate that there in nothing magical about any specific cut-off point before declaring that a result is important. A P value below a defined cut-off level has impact value but it is the size of the effect that has clinical significance. In this analysis, the method of analysis is irrelevant to clinical practice. The basic conclusion is that the hazard ratio for death and recurrence is approximately 0.74 if chemotherapy is added and we can be 99.9% confident that this effect is small but real.
Agreements and disagreements with other studies or reviews
Current opinion based on other studies
GOG 122 was the first adjuvant study in endometrial cancer to claim a significant benefit for adjuvant chemotherapy. However, controversy over the data analysis and control arm meant that the published conclusions have not been adopted as standard international practice. The early trial presentation was associated with the statistically significant effect seen only in a secondary analysis of the data, adjusted for perceived stage imbalance between the trial arms.The argument has been made that the chemotherapy arm had worse prognosis patients (presumably the Stage pT3c, however, lymph node involvement was not a prognostic variable in the study itself). The whole abdomen arm also had bad prognosis patients (pT3a with serosal/adnexal metastases). When the raw results are analysed, the difference in overall survival seen is not reflected in progression-free survival, which was not different between the arms. The statistical rationale for adjusting outcomes remains questionable. The history behind the trial begins with GOG 94 and GOG 107. GOG 94 was an observational cohort study of 180 women after maximally resected advanced endometrial carcinoma treated by adjuvant whole abdominal irradiation. The trial showed “tolerable toxicity”. The frequency of severe or life-threatening adverse effects among 174 patients evaluable for radiation toxicity included 12.6% with bone marrow depression, 15% GI, and 2.2% hepatic toxicity. GOG 107 was an observational cohort study representing the other arm of GOG 122. Doxorubicin 60 mg/m2 or doxorubicin 60 mg/m2 plus cisplatin50 mg/m2 was given every 3 weeks until disease progression, unacceptable toxicity, or a total of 500 mg/m2 doxorubicin. These toxicities and unconventional treatment regimens meant that the GOG 122 results were not adopted universally by the oncology community.
The presentation of the NSGO data has been more persuasive. The data showed that adjuvant platinum based combination chemo-therapy could reduce the risk of relapse with conventional statistical significance. Radiotherapy fields and doses were within those advocated by current guidelines. Overall survival was improved. The 93% certainty (probability = 0.07) of an effect from this single trial is strengthened by the recent supporting data from the EORTC and Italy ( NSGO, MaNGO and EORTC).
Review articles now tentatively promote the use of chemotherapy after hysterectomy for endometrial cancer if several poor prognosis features are seen on the hysterectomy specimen. The comprehensive review by Hogberg 2008 explores the possible conclusions from the trials and makes the point that the J GOG 2033 and Italian GICOG trial need to be interpreted with caution because the study populations are heterogenous and there is no agreement on the optimal chemotherapy regimen. It is possible that any advantage for either treatment option might have been hidden by adverse events in the low risk population. This same phenomenon was a major flaw in some of the radiotherapy trials. These trials grouped all subjects together with the consequence that a possible survival benefit in women with micro metastatic disease was hidden by mixing the summary plots with data from women with a low risk cancer. In other words, the survival advantage from adjuvant treatment may have been obscured in the summary plots because the data were mixed with women who were very unlikely to have micro metastases. In addition, some chemotherapy regimens involved outdated, non platinum regimens or low dose doxorubicin (Adriamycin) combinations such as 40 mg/m2 plus cisplatin 50 mg/m2 plus either 333 mg/m2 or 600 mg/m2 of cyclophosphamide every four weeks. In contrast the GOG 122 showed an advantage to chemotherapy and used up to seven cycles doxorubicin 60 mg/m2 plus up to eight cycles of cisplatin 50 mg/ m2 every three weeks. The NSGO trial also suggests an advantage to chemotherapy and used more modern regimens based on at least 50 mg/m2 of cisplatin or AUC 5 carboplatin with a second agent, either 175mg/m2 paclitaxel, 50 mg/m2 doxorubicin or other agent, usually every three to four weeks. It seems that the greatest advantage from chemotherapy comes from a higher dose regimen. However, not all reviewers agree. Stanojevic 2008 is cautious and concludes that there is insufficient evidence to base decisions with confidence, insufficient data to know who should benefit from adjuvant treatment and no good data to inform the clinician about the optimal regimen. Creutzberg 2010 agrees and is worried about the toxicity. Amant 2007 comments that the optimal regimen is not known but promotes doxorubicin and cisplatin combinations whilst acknowledging the rising promise of paclitaxel. Both Obel 2006 and Rodriguez 2008 support the view that Doxorubicin-cisplatin is still the standard chemotherapy regimen used in many centres despite the promise of paclitaxel-containing regimens and feel that adjuvant chemotherapy appears to be more effective than radiotherapy for type 1 cancers. Kodama 2007b and Gadducci 2007 are also encouraged by the data supporting the role of adjuvant chemotherapy and remind us that taxane platinum combinations are easier to tolerate and probably just as effective in advanced disease as the traditional more toxic regimens, Consequently it seems sensible to choose the less toxic option. The Japanese reviews are even more positive, presumably because of the influence of J GOG 2033. Hayakawa 1995 advocates doxorubicin plus platinum for women with the following factors: G3; invasion to > 1/2 myometrium; metastases to pelvic or para-aortic lymph node, isthumus-cervix extension; surgical stage III and the addition of cyclophosphamide for the poorer prognostic group. A German review (Fehr 2006) agrees that the anthracyclines, the platinum salts and the taxanes are all options and concludes (based on experience with metastatic disease) adjuvant chemotherapy can reduce recurrence rates from high-risk disease.
As well as expert opinion based on randomised trials, the literature contains reports of useful comparative studies. Unfortunately, they lack a well-designed control arm and this limits any conclusions that can be drawn but they are still worthy contributions. Hirai 2002 assessed 251 women treated with chemotherapy after hysterectomy for stage I endometrial cancer. Fifty-four women were picked out for analysis because they had lymph-vascular space invasion. There was a statistically significant difference in the 10 year overall survival favouring women receiving a variety of adjuvant chemotherapy agents (P = 0.02). Aoki 2004 described 170 women with FIGO stage I and II endometrial cancer. Forty-one received adjuvant cyclophosphamide, doxorubicin and cisplatin. When stratified into low-risk and high-risk groups based on a score system of prognostic factors (histologic grade 3, invasion of the outer half of the myometrium, lymph-vascular space invasion, and cervical invasion) the five-year disease-free survival and overall survival rates were significantly better if women with high-risk disease received adjuvant chemotherapy. Kodama 2007a’s study of 167 women with surgically staged IB-II and IIIA (positive peritoneal cytology only) cancers included 58 patients (34.7%) who received cyclophosphamide, epirubicin and cisplatin or paclitaxel, pirarubicin and carboplatin. Adjuvant chemotherapy was administered to 14 of 23 patients with histologic grade 3 tumours. Their five-year overall survival rate was 92.3%, significantly higher than the rate for women who had not received chemotherapy (50.0%). These studies imply that adjuvant chemotherapy might improve the survival in early-stage endometrial cancer patients with high-risk factors. However, the lack of a well-designed control arm and the obvious worry that the women who did not receive chemo-therapy did not because they declined the additional treatment or were simply not fit enough for it means that the difference between the groups might have happened anyway. Nevertheless, it does offer some support to the belief that adjuvant chemotherapy has useful activity. Further but very limited support comes from similar non-randomised comparative studies. The prequel to J GOG 2033 included the Japanese report of 81 women with high-risk endometrioid adenocarcinoma after hysterectomy who had undergone adjuvant chemotherapy with cisplatin (50 mg/ m), doxorubicin (35 mg/m2), and cyclophosphamide (500 mg/m2 given every three weeks. Data from 67 women who received whole pelvic irradiation with or without para-aortic irradiation were retrospectively examined as historical controls. The five-year overall survival rate for women receiving chemotherapy was 87% compared to 82% compared to the historical controls who received radiotherapy group (P = 0.1). Again bias means that the data have limited analytical value but the trend supporting advocates of chemotherapy is persistent.
The studies above represent some of the evidence to suggest that chemotherapy might have a role as a useful adjuvant therapy. However, the non-randomised comparative studies can do nothing more than suggest a role for chemotherapy. The comparative studies may not compare outcomes from similar groups and any reader must be left wondering how women were selected to receive the allocated treatment. The obvious worry about conclusions from the non randomised comparisons is that they study fitter women who can tolerate the more toxic treatment and their improved survival is because they were fitter. This is why conclusions about the efficacy of treatment are best based on a systematic analysis of randomised trials. However, the conclusions of this systematic review concord with all the other modern philosophers and this strongly suggests that we need to change practice and consider offering chemotherapy to selected women with high-risk disease after hysterectomy, either with or as an alternative to radiotherapy.
What this review adds
This systematic review also explored all the review papers from 2004 onwards. The strong consensus from these reviews is that chemotherapy has activity in endometrial cancer. There is no value in using it for women with a low risk of recurrence as they receive the toxicity of treatment and they only gain a small potential reduction in a risk that is already low. The harm will probably exceed the benefit. However, when the risk of recurrence is high, the potential advantage of treatment increases and this benefit may overtake the harm from the treatment toxicity. Multiple agents, higher doses and longer and more frequent courses add to treatment efficacy but there is a diminishing return. As the gain from adjuvant therapy is likely to be small and as many women with endometrial cancer have co-morbidities, the focus must be to move away from the traditional and relatively toxic combination of doxorubicin plus cisplatin plus cyclophosphamide to carboplatin with or without a taxane. The data in this review cannot add to the debate on the correct regimen as there are insufficient studies in adjuvant treatment. Fujimura 2000 and Deng 2000 offer some insight but neither used the taxane-platinum option. We may know more about the added value of a taxane when the US Gynecologic Oncology Group (GOG) and the Japanese Gyneco-logic Oncology Group (J GOG) report their randomised studies exploring taxane-platinum regimens. This review can only confirm that chemotherapy after surgery may have some activity and this may translate into either a survival advantage, longer disease free interval and a change in the pattern of recurrent disease.
The inconvenience and cost of chemotherapy are undeniable. They can be evaluated by experience and do not need quantification by a supporting randomised trial. Therefore it probably does not matter that there is no trial evaluating the option of carboplatin or platinum -taxane. The toxicity is known from experience and can be estimated for each patient by any experienced oncologist. What matters is that platinum based chemotherapy works, the gain is greatest in women with a higher risk of relapse and in those with a potentially long future ahead of them. An oncologist can now say that the benefits are real but small and therefore the chemotherapy regimens need to be individualised.
They may be a 7% survival advantage for high-risk women when four cycles of modern chemotherapy are added to radiotherapy. Some women might also find it a preferable alternative to radiotherapy.
AUTHORS’ CONCLUSIONS
Implications for practice
This analysis suggest that adjuvant chemotherapy can reduce the risk of recurrent disease and improve the chance of surviving endometrial cancer. The analysis focusing on modern chemotherapy regimes suggests that 20 women required treatment in the trials for one to survive. The data also suggests that chemotherapy reduces the risk of death at any specific time by a quarter compared to not receiving it. We do not know who will benefit from chemotherapy or what drugs regimes to use but it seems logical to assume that most of the benefit is achieved with up to 4 course of platinum based therapy and the woman who gains most is the one with the highest risk of developing a distant metastasis.
Most women with few risk factors are already cured. The cost and harm from adjuvant therapy to this group exceeds the benefits even though a few will eventually suffer recurrent disease. The cost and toxicity for women with a high risk of recurrence will be the same but more women will have undiagnosed micrometastases after surgery and therefore more will be cured by adjuvant therapy. Concider an intermediate low risk cancer with a 4% risk of distant recurrence. Chemotherapy will only change her cancer death rate by 1% (HR=0.76). However, high risk cancer may have a 40% risk of recurrence. In this scenario, chemotherapy could benefit one in 10 by reducing the risk of cancer progression and death from 40% to 30%.
The difficult decision for the oncologist concerns the treatment regimes and patient selection. The survival advantage can be relatively small and there is a diminishing return from long courses of toxic combination regimes. The difficult issue for the woman with cancer is balancing the toxicity against the survival advantages and her natural life expectancy. Chemotherapy will reduce the risk of recurrent disease, increase her time before a recurrence and increase her overall survival chances. However, this needs to be traded against the side effect profiles. The combination of adjuvant radiotherapy and chemotherapy should be considered for women with a very high risk of metastatic disease who would otherwise have a long life expectancy. Another option is to use chemotherapy with brachytherapy as an alternative to pelvic radiotherapy.
Implications for research
This analysis makes it difficult to justify repeating the trials of chemotherapy versus no other treatment. The answer is clear. We now know that adjuvant chemotherapy works. The focus will now be on making chemotherapy more tolerable but this is not a matter for randomisation techniques. Most platinum based doublets are iso-effective and the effect of a third agent is likely to be so small that it could not be detected in any conventional sample size randomised trial. Cohort studies teach us about toxicity and tolerability and randomised trials of different regimens will not add any new revolutionary facts that will radically alter practice. Furthermore, the research dollar would be better spent elsewhere. The remaining questions that need randomised trials in this focused field related to concomitant chemoradiation and radiotherapy fields. Chemoradiation is clearly more effective than radio-therapy for cervix cancer and this needs to be tested against sequential radiotherapy and chemotherapy for very high-risk early endometrial cancer. We also need to compare and contrast the toxicity of brachytherapy combined with chemotherapy against the toxicity of sequential radiotherapy and chemotherapy.
PLAIN LANGUAGE SUMMARY.
The effect of chemotherapy on survival from early womb cancer after hysterectomy
Womb (uterine/endometrial) cancer is a fairly common disease affecting approximately 1 in 70 women. A hysterectomy is usually curative because most cancers have a low risk of spreading (metastasising) to other sites which may result in a later recurrence. Microscopic examination of the hysterectomy specimen can tell doctors if there is a high risk of the cancer returning and this allows women to decide if they want further preventative treatment (adjuvant therapy) to reduce the risk. Chemotherapy can increase cure rates for other types of high-risk cancer after initial surgery and this review examines the effectiveness of chemotherapy for primary womb cancer after hysterectomy. Data from nine high quality randomised clinical trials involving up to 2197 women were subjected to systematic statistical modelling. This shows that chemotherapy reduces the risk of recurrent disease, lengthens the duration women have before a metastasis is diagnosed and improves survival rates. There are many ways to examine the data. The subset analysis that excluded old fashioned drug regimens suggests that chemotherapy reduces the risk of being dead at any nominated time by a quarter. The number of women who would need to have need chemotherapy to prevent one death depends on the type of cancer. In these trials, one woman was cured for every 25 women treated with high dose platinum based chemotherapy after hysterectomy. This is an absolute risk reduction of 4%. Chemotherapy is associated with a greater survival advantage than radiotherapy and has added value when used with radiotherapy. It also appears to reduce the absolute risk of developing a recurrence outside the pelvis by about 5%. This would benefit one woman in every 20 treated. However, chemotherapy has side effects, risks and temporarily reduces a woman’s quality of life. In many cases, the small reduction in the cancer recurrence risk may not be worth the side effects of adjuvant treatment.
Figure 11.
Treatment hazard ratio from GOG 122 with 95% CI by prognostic group and end point. (A) Progression-free survival; (B) overall survival. AP, doxorubicin and cisplatin; WAI, whole-abdominal irradiation; HR, hazard ratio; Relative Hazard, treatment hazard ratio estimate; Var[In(HR)], variance of the log treatment hazard ratio estimate.
ACKNOWLEDGEMENTS
We thank Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [author-defined order]
| Methods | The collaborative Nordic and European study randomly studied the effect of adding a platinum based chemotherapy regimen to hysterectomy and radiotherapy for women with surgically excised endometrial cancer | |
| Participants | The latest report provides data on 63 women from 12 EORTC centres. The eligibility criteria included histologically proven endometrial cancer of any type and infiltration to more than half the myometrial thickness. Women with positive peritoneal cytology and/or positive pelvic lymph nodes were also eligible and participants must have been fit to receive combination chemotherapy, have a WHO performance status of 0 to 2, adequate bone marrow, renal, hepatic and pulmonary function and give informed consent | |
| Interventions | Early participants in the chemotherapy arm received four courses of cisplatin = 50mg/m2 + doxorubicin 50mg/m2 or epirubicin 75mg/m2. Later in the trial, several regimens were allowed, of which cisplatin + doxorubicin, paclitaxel 175mg/m2 + epirubicin 60mg/m2 + carboplatin AUC 5, and paclitaxel 175mg/m2 + carboplatin AUC 5/6 were most popular The control group were treated by pelvic radiotherapy ± vaginal brachytherapy to a dose of 44 Gray |
|
| Outcomes | The primary outcome was relapse free survival. The secondary endpoints were:
|
|
| Results (OS & PFS) | see the combined EORTC and NSGO table | |
| Results (site of recurrence) | see the combined EORTC and NSGO table | |
| Toxicity | see the combined EORTC and NSGO table | |
| Notes | The data are still immature and the survival curves will evolve as later recruiters reach 5 years survival. The EORTC have not yet published the full report but early data are available from ASCO presentations and a report including the MaNGO trial is in the 2010 European Journal of Cancer | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was organised by the EORTC headquarters using a minimisation procedure with the stratification factors for each centre |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal treatment allocation from the participants but the treatment allocation will have been concealed from the data analysts |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The data collection was by the EORTC data centre, an organisation of the highest scientific standard |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | The trial has not yet been reported but the data will be analysed by the EORTC data centre, an organisation of the highest scientific standard |
| Selective reporting (reporting bias) | Low risk | The trial has not yet been reported but the data will be analysed by the EORTC data centre, an organisation of the highest scientific standard |
| Other bias | Unclear risk | The main issue are that details of the study population have not yet been formally published |
| Main problems with the trial as perceived by this analysis | Unclear risk | The trial has not yet been reported |
| Methods | NSGO-EC-9501 (in collaboration with EORTC −55991 - amendment 1) was a phase III open, randomised I national clinical trial | |
| Participants | 320 women from 13 Nordic centres with surgical stage I, II, IIIA (positive peritoneal fluid cytology only), or IIIC (positive pelvic lymph nodes only) endometrial cancer participated. Women had histologically proven endometrial cancer of one of the following types: FIGO grade 3 (poorly differentiated endometrioid adenocarcinoma) with infiltration ≥ 50% of the myometrial thickness or any serous, clear cell or anaplastic carcinoma and women had to have had an endometrial cancer confined to the corpus cervix, regional lymph nodes or peritoneal fluid cytology. They were treated by hysterectomy, bilateral salpingo-oophorectomy and external beam radiotherapy. The trial recommended CT-based computer-aided 3-dimensional dose planning and four field technique. The prescribed dose to the target volume was at least 44 Gray. If other fractionations than 2.0 Gray five times per week were used, the dose was converted to a 2 Gray equivalent dose according to the linear quadratic formula. Brachytherapy was included as a part of the standard radiotherapy procedure in some centres and the decision to deliver brachytherapy was made before randomisation Women were excluded if there was:
|
|
| Interventions | Women treated by Surgery and radiotherapy were allocated no additional treatment or chemotherapy. Chemotherapy was given before or after completion of the radiation. The decision about sequence had to be taken before randomisation. The following combinations were used: AP: Doxorubicin 50 mg/m2 (epirubicin 75 mg/m2) and cisplatin 50-75 mg/m2 q 3 weeks. Cisplatin could be substituted with carboplatin AUC 5-6. TP: Paclitaxel 175 mg/m2/3 hours and carboplatin AUC 5-6 q 3 weeks. TEC: Paclitaxel 175 mg/m2/3 hours, epirubicin 60 mg/m2 (doxorubicin 40 mg/m2), and carboplatin AUC 5 q 3 weeks TAP: Doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 day 1, paclitaxel 160 mg/m2/3 hours day 2, G-CSF 5 μg/kg/day starts on day 3 and continues for at least 10 days q 3 weeks (GOG-177 (46)). At least four cycles were planned. |
|
| Outcomes | The primary end-point was to compare progression-free survival of patients treated with either radiation alone or radiation and chemotherapy given sequentially. The secondary end-points were overall survival, toxicity and pattern of progression |
|
| Results (OS & PFS) | see the combined EORTC and NSGO table | |
| Results (site of recurrence) | see the combined EORTC and NSGO table | |
| Toxicity | see the combined EORTC and NSGO table | |
| Notes | For follow-up, patients were seen 3 and 6 months after end of treatment, thereafter every 6 months for 5 years. Survival was calculated from the date of randomisation until the date of death. The progression-free survival was calculated as time elapsed from date of randomisation to date of progression or death of disease, whichever was the first registered event. Patients who died of non-disease-related causes were censored at time of death. Toxicity was graded according to CTC 1999 | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was performed centrally by the study office at Linkoping University Hospital with stratification for centre and histology |
| Allocation concealment (selection bias) | Low risk | The data analysis was organised by the data centre at the NSGO. Although the statistician’s awareness of the group allocation has not been formally stated, it will have been of the highest quality and will have observed the usual rules |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It was not possible to conceal the treatment allocation from the participants |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | The trial has not yet been reported |
| Selective reporting (reporting bias) | Low risk | There is no suggestion of selective reporting |
| Other bias | Low risk | There is no suggestion of bias |
| Main problems with the trial as perceived by this analysis | Low risk | The main issues are that the study population were a heterogenous group with very variable risk factors including some women with grade 1 disease, some with clear cell cancers and some with stage 3 |
NSGO & EORTC
| Methods | See the tables for each study | |
| Participants | The pivotal presentation at the 2007 ASCO meeting was the earliest report. This describes 382 women. 12% had stage 1a (intra endometrial/noninvasive cancer) 26% stage 1b, 49% stage 1c, 6% stage 2 and 1.6% stage 3 cancer. 27% had grade 1 or 2 cancer, 53% had grade 3 endometrioid cancer, 14% had grade 3 serous papillary or clear cell morphology and more data are needed on the other 6%. Most women had two or more of the risk factors: grade 3, deep myometrial invasion, or DNA non-diploidy. 378 women are described in the report published in the European Journal of Cancer. This report adds that brachytherapy was used in less than half the cases and unfortunately, we do not know if lymphadenectomy was performed in two thirds of cases | |
| Interventions | See the tables for each study | |
| Outcomes | See the tables for each study | |
| Results (OS & PFS) | The latest report combined the results from the two trials and includes data on 378 women, 191 randomised to radiotherapy only and 187 allocated chemotherapy with radiotherapy. The HR for overall survival depending on randomisation (intention to treat) = 0.66 (95% CI; 0.40-1.08; P = 0.10). The hazard ratio for progression-free survival depending on randomisation after censoring deaths from intercurrent causes was 0.64 (95% CI; 0.41-0.99; P = 0.04) | |
| Results (site of recurrence) | The latest report quotes disease progression in 46/191 (24%) radiotherapy arm 28/187 (15%) in the radiotherapy with additional chemotherapy arm. Therefore, it is best to rely on the latest report and accept that data added together from 3 trials, MaNGO and Hogberg. This quotes a rate of initial recurrence in the pelvis 11/267 for women allocated radiotherapy versus 5/267 for women allocated radiotherapy with additional chemotherapy. This number of distal and multiple sites of recurrence for women allocated radiotherapy was 52 and 7 respectively from 267 women allocated radiotherapy only and 35 and 3 respectively from 267 women allocated radiotherapy with additional chemotherapy | |
| Toxicity | There was one treatment-related death 3 months after randomisation in a woman allocated no chemotherapy. No further details are available. There were eight serious adverse events in the chemotherapy arm. Two women had diarrhoea, one combined with neutropenia. Three women had neutropenia, one acquired pneumonia requiring respirator treatment, and another with associated nausea and vomiting. One woman had an allergic reaction to paclitaxel, one had an episode associated atrial fibrillation and one developed bilateral pulmonary emboli 24 days after the first cycle There was one serious adverse event in the no chemotherapy arm; an intestinal reaction with diarrhoea which led to cessation of radiotherapy after 36 Gray All serious adverse events resolved after appropriate treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation was employed in accordance with good practice |
| Allocation concealment (selection bias) | Low risk | Analysis was by statisticians who had treatment allocation concealed |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It was not possible to conceal the treatment allocation from the participants |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | The compliance to radiotherapy was high, 182/191 (95%) and 178/187 (95%) received P44 Gray in the radiotherapy arm and radiotherapy-chemotherapy arm, respectively. Of the 187 patients assigned to chemotherapy, 136 (73%) received four treatment cycles as planned. Eighteen (9.6%) received no chemotherapy and data were not available from three women |
| Selective reporting (reporting bias) | Low risk | The trials have been reported in accordance with the Consort criteria |
| Other bias | Low risk | There is no suggestion of any bias |
| Main problems with the trial as perceived by this analysis | High risk | Data collection is still ongoing and the data from the trials have been merged, rather than reported separately |
| Methods | The Mario Negri Gynecologic Oncology group (MaNGO) collaborative clinical gynaecologic oncology group from 20 centres, mainly in the northern part of Italy organised ILIADE-III, a phase 3 randomised trial | |
| Participants | The latest report describes 157 women with histologically confirmed endometrioid carcinoma, FIGO 1988 stage IIB, IIIA-C disease (stage IIIA with positive cytology alone without other risk factors was not included). They were excluded if the pathology was serous or clear cell carcinomas, if the performance status > 2. All women had a hysterectomy and removal of tubes and ovaries and lymphadenectomy was performed in about half (54%) of the cases. Pelvic radiotherapy involved 1.8 Gray fractions up to a total dose 45 Gray. The field was extended to L1/2 if there was a para-aortal metastases. Vaginal brachytherapy was added for women with cervical stromal involvement. | |
| Interventions | Women were randomised to chemotherapy or no further treatment after hysterectomy and radiotherapy. Chemotherapy had to start within 30 days after surgery and consisted of doxorubicin 60mg/m2 + cisplatin 50 mg/m2 every 3 weeks for three cycles. The interval between chemotherapy and radiotherapy was less than 4 weeks. Women who did not receive chemotherapy had radiotherapy commenced within 40 days after surgery | |
| Outcomes | The primary end-point was PFS. All times were counted from the time of randomisation. PFS was defined as the time to progression of endometrial cancer or death from all causes. Secondary end-points were OS; the time to death of all causes and cancer-specific survival (CSS); the time to death related to endometrial cancer. Survival differences between groups were expressed as hazard ratios and were analysed with univariate Cox proportional hazard models | |
| Results (OS & PFS) | The overall survival appeared better but not significantly so in favour of women allocated additional chemotherapy The HR (95% CI) was 0.74; 0.36 to 1.52, representing 17/76 (22%) reported deaths with no chemotherapy versus 14/187 (18%) if is chemotherapy was used Similarly, the progression-free interval appeared better but not significantly so in favour of women allocated additional chemotherapy. The HR (95% CI) was 0.61; 0.33 to 1.12, representing 26 (34%) reported deaths with no chemotherapy versus 18 (23%) reported events if chemotherapy was used |
|
| Results (site of recurrence) | Disease progression for women in the MaNGO-trial were 24/76 (32%) and 15/80 (19%) | |
| Toxicity | There were no treatment related deaths. Toxicity data were only available from 74 women. 12/74 (16%) developed grade 3/4 leucopenia, 4 (5%) suffered grade 3/4 nausea and vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally by telephone at the Mario Negri Institute, Milan in blocks that balanced the treatment assignment within each site |
| Allocation concealment (selection bias) | Low risk | Randomisation was centrally organised and data were analysed using the intention-to-treat principles |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It is not possible to conceal the treatment allocation from the participants. The trial was supervised by the GOG Data Monitoring Committee without knowledge of treatment results |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | The data have been reported in line with Consort guidance. For example we know that 76 women were allocated radiotherapy, 67 received it, 4 did not and the data are missing on a further 5 cases. Of the 80 allocated chemotherapy and radiotherapy, 71 Completed 3 cycles, 3 received less than 3 cycles, 2 did not receive any chemotherapy and data are missing fro 4 cases. Also of the 80, 74 received radiotherapy, 4 did not, data are missing on 2 cases and 2 further cases in this group had neither chemotherapy nor radiotherapy |
| Selective reporting (reporting bias) | Low risk | There is no suggestion of selective reporting. |
| Other bias | Low risk | Earlier presentations quote slightly different figures from the later ones but the differences are small and probably reflect maturity in the data rather than counting errors |
| Main problems with the trial as perceived by this analysis | Low risk | Like many of these studies, MaNGO recruited women with a variety of stages of disease, variety of surgical techniques and some of the data are missing. Overall, the quality of this trial was high and it has been coherently reported |
| Methods | After surgery, women were randomised centrally by the Finnish Cancer Registry to two groups: Both groups received pelvic radiotherapy. Group A had no additional treatment. Group B was given chemotherapy during gaps in radiation treatment. The first dose of chemotherapy was given 1 to 2 weeks after surgery and before radiotherapy. The second cycle was given after half of the radiotherapy had been given. The third treatment cycle followed 2 weeks after the radiotherapy | |
| Participants | The study population consisted of 156 women with endometrial cancer treated in four Finnish university hospitals between April 1992 and April 1996. They were followed up until April 2001, Women had noninvasive or early myoinvasive (< 50%) grade 3 endometrial cancer or any grade cancer that was deeply myoinvasive (> 50%), involving the cervix or peritoneal washings (old FIGO stage 1c-lllA). Thirteen (18%) women allocated radiotherapy only had stage 2 disease compared to 21 (25%) women allocated chemotherapy with radiotherapy. Nine (12.5%) women allocated radiotherapy only had malignant cells in the pelvic washings compared to 10 (11.9%) of women allocated chemotherapy with radiotherapy. The rest had stage 1 disease The surgery included a total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy. 80% of women also had pelvic lymphadenectomy |
|
| Interventions | Women allocated only radiotherapy received a total dose of 56 Gray (2 Gray/fraction) delivered to the pelvis using a four-field technique. The treatment was divided in two courses 28 Gray each, separated by a pause of 3 weeks. The first course started 4 to 5 weeks following the operation Women allocated chemotherapy with the radiotherapy received three chemotherapy cycles consisting of cisplatin 50 mg/m2 Cisplatin, epirubicin 60 mg/m2 and cyclophosphamide 500mg/m2. The first cycle was given after the final histopathological report was available, or 1 to 2 weeks after the operation. The second one was given during the pause in radiotherapy. The third course was given within 2 weeks after the completion of the second radiation course |
|
| Outcomes | The primary outcome was 5 year survival rate. | |
| Results (OS & PFS) | During the five-year follow-up time 32 patients (20.5%) had recurrent disease. The recurrence rate was 22.6% in women allocated chemotherapy with radiotherapy and 18.0% in women allocated radiotherapy only. The difference is not statistically significant (P = 0.50) As calculated from the operation data, the median time to recurrence was 20 (range 8 to 60) months in women allocated chemotherapy compared to 15 (range 6 to 37) months in women allocated radiotherapy only (P = 0.17) Of the 32 patients with recurrent disease, only 6 stayed alive for the rest of the follow-up time. Twenty-six (81.2%) died of endometrial carcinoma. The median time interval from the recurrence to death was nine (range 2 to 22) months for women allocated chemotherapy with radiotherapy and eight (range 3 to 11) months for women allocated radiotherapy only. Even among the surviving patients, only two (one in each group) with a local recurrence were free of disease at the end of the follow-up. Fifteen women allocated chemotherapy with radiotherapy died and 11 of the women allocated radiotherapy only died. Consequently the disease-specific five-year survival was 82.1% and 84.7% respectively. The median survival for women allocated chemotherapy with radiotherapy was 37 (range 13 to 50) months compared to 23 (range 15 to 44) months for women allocated radiotherapy only (P = 0.148). The median disease-free survival was 25 months (range 12 to 49) for women allocated chemotherapy with radiotherapy and 18 (range 9 to 36) months for women allocated radiotherapy only (P = 0.13) |
|
| Results (site of recurrence) | The Kuoppala 2008 used chemotherapy sandwiched in between the radiotherapy courses. This trial reported a pure locoregional recurrence rate of 3.2% evenly distributed in the two groups, 3 women treated without chemotherapy of which 2 were alive after 5 years of follow up versus 2 treated by chemotherapy, of which one was alive after 5 years. In this trial chemotherapy did affect the distal recurrences rate, 13.8% versus 20.2%. Ten women (all died) developed a distal recurrence in the group not allocated chemotherapy compared to 17 (3 were still alive after 5 years of follow up) if chemotherapy had been used | |
| Toxicity | Eight allocated both radiotherapy and chemotherapy required laparotomy for intestinal obstruction compared to 2 women treated by radiotherapy only Two patients in both groups experienced other major complications. One woman allocated chemotherapy with her radiotherapy suffered ureteric obstruction and one developed pelvic bone necrosis and bowel fistulation. One woman allocated radiotherapy only developed multiple gastric ulcerations causing serious malnutrition and another perforated her sigmoid colon. This was not attributable to recurrence Chemotherapy was implemented in three courses. The first, second and third treatment course could be administered according to the protocol 79 (94.0%), 70 (83.3%) and 64 (76.2%) women respectively. Of these, 6, 13 and 14 women respectively experienced grade 3 to 4 leucopenia. None suffered from grade 4 infection. There were two cases of sepsis and eight had Grade 3 infections. Nausea and vomiting were assessed as grade 3/4 in 5.6%. Grade 3 and 4 diarrhoea did not occur. Alopecia was severe (grade 3/4) in 49% of women. Liver and kidney functional test results remained within normal range throughout the chemotherapy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were allocated treatment by the central Finnish Cancer registry |
| Allocation concealment (selection bias) | Low risk | Allocation was coded by group A or B. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It is not possible to conceal the treatment allocation from the participants |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Data were analysed on an intention to treat basis and all cases are accounted for In the group allocated chemotherapy with radiotherapy, 78 women (92.9%) were treated according to the protocol during the first period. Owing to intestinal symptoms one woman received only 20 Gray and another 22 Gray. One was treated with 49 Gray and another with 50 Gray in one period of radiotherapy. Two patients were not treated at all. In addition one patient had intestinal symptoms and one had infection, but both were nevertheless treated fully. During the second period the treatment was successful in 77 of women in this group (91.6%). Three had low leucocyte levels but were nevertheless treated according to protocol. Four patients complained of intestinal symptoms. Of, these, three were treated to the full dose but one received only 14 Gray. The second radiation period was omitted for one patient, because she developed sepsis. One refused the second treatment and four received none at all for unknown reasons Not all women received the prescribed radiotherapy dose, For women allocated radiotherapy only, 68 women (94.4%) received the full dose. One patient received 36 Gray and one was treated in one treatment period only with a dose of 45 Gray. Two patients refused the treatment and received no radiotherapy at all. During the second period 66 patients (91.6%) of the women allocated radiotherapy only were treated according to the protocol. Three suffered from intestinal symptoms but were treated to the full dose. However, there were another three patients treated to a suboptimal dose They received 11, 18 and 21 Gray, respectively. One patient was treated only during the first period and two patients opted to receive no radiotherapy at all |
| Selective reporting (reporting bias) | Low risk | There is no suggestion of selective reporting but that trial is old and was not reported according to CONSORT criteria |
| Other bias | Low risk | The groups were well matched. |
| Main problems with the trial as perceived by this analysis | Unclear risk | The trial design split the radiotherapy to include a break in treatment after delivering 26 Gray. This may allow metastatic deposits to recover especially in the group allocated radiotherapy. The split regimens would not be used in modern oncology and this limits the relevance of the data from this study |
| Methods | This was a GOG multicenter RCT. Women who had completed adjuvant radiotherapy, were randomised to receive no addition treatment or doxorubicin | |
| Participants | 224 women entered the study, 112 in each arm. Forty-three were deemed ineligible on review of the submitted data (appropriately so in our opinion) leaving 181 cases for evaluation. The original entry criteria were defined as primary, previously untreated, histologically confirmed invasive carcinoma of the endometrium stage I and II (occult), all histologic grades, with one or more of the following high-risk features: (1) equal to or greater than 50% myometrial invasion; (2) histologically documented pelvic or aortic node metastases; (3) histologically documented cervical extension without gross clinical evidence of cervical involvement; (4) adnexal metastases. All patients were required to undergo total abdominal hysterectomy, bilateral adnexectomy, selective pelvic and aortic lymph node dissection, and peritoneal cytology. Radiation therapy was started within 6 weeks of surgery. Women without aortic node metastases receive 5000 rads external beam therapy to the whole pelvis at 160 to 180 rads per day to a point 7 cm lateral to the midline of the pelvis at the widest transverse diameter. An 8 cm wide aortic field extended to the top of T10 was added to the pelvic field if there were para aortic lymph node metastases. The target dose to the aortic port was 4500 rads. Intracavitary therapy was not permitted | |
| Interventions | After completion of radiation therapy, the patients were randomised to receive doxorubicin bolus therapy (60mg/m2 starting dose) to a maximum cumulative dose of 500 mg/m2 (although the methodology quotes 400mg/m2 as the earlier upper limit, amended during the trial to 500mg/m2 Between November 1977 and July 1986, 92 women were randomly allocated doxorubicin and 89 women were allocated no additional therapy entered the no-DOX arm |
|
| Outcomes | The primary outcomes investigated in this trial were survival and disease free interval | |
| Results (OS & PFS) | The five-year survival rates for women with deep myometrial invasion, cervical involvement, and pelvic node metastases were similar (63% to 70%), whereas the rate for patients with aortic node metastases was 26%. The hazard ratios were reconstructed from the survival curves | |
| Results (site of recurrence) | GOG 34 reported 16.3% of women (15/92) allocated to Doxorubicin had extrapelvic recurrence compared to 22.5% (20/89) not given chemotherapy. Data on the risk of pelvic recurrence is not available but it is said that there is no difference in rates | |
| Toxicity | There were no cases of grade 3 or 4 cardiac toxicity. Twelve women (6.9%) developed small bowel obstruction after radiation therapy. There were three treatment related deaths in the group allocated doxorubicin chemotherapy and two in the radiotherapy-only arm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | GOG statistical office supervised treatment allocation |
| Allocation concealment (selection bias) | Low risk | GOG statistical office supervised the analysis |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It is not possible to conceal the treatment allocation from the participants. The trial was supervised by the GOG Data Monitoring Committee without knowledge of treatment results |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | This study was closed to patient entry in July of 1986 after concluding that successful completion was highly unlikely because of the reduced accrual rate and the surprisingly good recurrence-free survival rates of all but one high-risk category. In addition, the study was said to be flawed by the relatively large number of patients on the experimental regimen who, after randomisation, declined to receive any doxorubicin |
| Selective reporting (reporting bias) | Low risk | There is no suggestion of selective reporting but that trial is old and was not reported according to CONSORT criteria |
| Other bias | High risk | The most obvious imbalance is the higher frequency of pelvic node metastases and G3 lesions in the group allocated doxorubicin. Compliance in this study was a problem. Of the 92 women randomised to receive doxorubicin, 25 did not receive doxorubicin and 2 received only one course. This was usually due to patient refusal |
| Main problems with the trial as perceived by this analysis | Unclear risk | The low number of women receiving adequate chemotherapy will affect the statistical outcome of this group |
| Methods | This was a GOG multi centre randomised trial comparing doxorubicin-cisplatin (AP) chemotherapy with whole-abdominal irradiation (WAI) in women with stage III or IV endometrial carcinoma having a maximum of 2 cm of postoperative residual disease. A total of 190 were randomly assigned to receive AP and 198 women were assigned to receive WAI | |
| Participants | Four hundred twenty-two women were entered between May 1992 and February 2000, of whom 388 were initially eligible. Women with FIGO stage III or IV endometrial carcinoma of any histology were eligible for this trial. Eligibility required total abdominal hysterectomy and bilateral salpingo-oophorectomy, surgical staging, tumour resection, no single site of residual tumour more than 2 cm and normal haematology and biochemistry. Nodal sampling was optional but women with para-aortic lymph nodes metastasis were required to have negative scalene node biopsies and chest computed tomography scans | |
| Interventions | Chemotherapy consisted of doxorubicin 60 mg/m2 plus cisplatin 50 mg/m2 every 3 weeks for eight cycles. The maximum allowable cumulative dose of doxorubicin was 420 mg/m2. Therefore only cisplatin was to be infused during the eighth cycle. Hydration was maintained by administering normal saline at 500 ml/h for 2 hours before and after the cisplatin dose. Doxorubicin doses were reduced based on pretreatment blood counts, with dose levels reduced from 60 to 15 mg/m2 in 15-mg/m2 increments. Doses were re instituted with recovery of myelosuppression. Treatment interruption caused by myelosuppression exceeding 6 weeks required discontinuation of protocol therapy Whole abdominal radiation therapy (WAI) was delivered with an open-field anterior-posterior/posterior-anterior technique with up to 30 Gray in 20 daily fractions. Kidney blocks were used posteriorly but there was no liver shield. After WAI, women received a boost to the true pelvis or to an extended field encompassing pelvic lymph nodes (PLNs) and para-aortic lymph nodes (PALNs). A boost to both areas was administered to patients with positive PLN and no PALN sampling or patients with neither PLN nor PALN sampling. Pelvic (± para-aortic) boosts were accomplished using a four-field box technique with custom blocking to minimise the treated small-bowel volume. The boost dose was 15 Gray in 8 fractions. All fields were treated once daily, 5 days per week |
|
| Outcomes | The primary end point for comparison of the treatment regimens was PFS; OS was a secondary end point | |
| Results (OS & PFS) | At the time of reporting the final analysis, 98 patients (51%) on the chemotherapy arm were alive compared to 76 women (38%) on the WAI arm. Women alive or deceased, without evidence of tumour progression included 97 (50%) and 93 (46%) in the AP and WAI arms, respectively, including 18 and 22 women, respectively, who died before documentation of tumour progression. The majority of deaths were attributed to cancer progression. The progression hazard ratio relative to the WAI arm, adjusted for stage, was 0.71 (95% CI, 0.55 to 0.91; P = .007). This adjusted relative hazard ratio was associated with a predicted increase in PFS at 60 months of 12% (50% for AP versus 38% for WAI). There was also a statistically significant difference in overall survival between the two arms. Subgroup analysis, presented at the IGCS in 2010 showed that this was independent of cervical stromal involvement of lymph node metastasis The hazard ratio of death relative to the WAI arm, adjusted for stage, was 0.68 (95% CI, 0.52 to 0.89; P = .004). This adjusted relative hazard estimate was associated with a predicted increase in survival at 60 months of 13% for patients on the AP arm versus WAI patients (55% versus 42%, respectively). Unadjusted Kaplan-Meier estimates of five-year PFS and OS were 42% and 53%, respectively, in the AP arm compared with 38% and 42%, respectively, in the WAI arm. The unadjusted hazard ratio recalculated from the survival curves of GOG 122 reveal that the overall survival hazard ratio is 0.74 (0.57, 0.96) and the progression-free survival hazard ration is 0.81 (0.63 to 1.05) Treatment hazard ratio with 95% CI by prognostic group is reproduced in Figure 11. |
|
| Results (site of recurrence) | The initial site of recurrence was limited to the pelvis in 27 women (13%), within the abdomen in 33 women (16%), and extra-abdominal or liver metastases in 45 patients (22%). Four women allocated whole abdominal radiotherapy and in two allocated chemotherapy arm, sites of initial recurrence were unknown. Of the 194 women given chemotherapy, 97 (50%) had documented tumour recurrence. The initial site of recurrence was limited to the pelvis in 34 patients (18%); 27 patients (14%) experienced disease recurrence within the abdomen, and, in 34 patients (18%), the first recurrence included extra-abdominal or liver metastases | |
| Toxicity | Comparisons of grade 3 to 4 haematologic toxicities between AP and WAI were: WBC (62% versus 4%, respectively), absolute neutrophil count (< 85% versus 1%, respectively), platelets (21% versus 3%, respectively), and maximum haematologic toxicity (defined as percentage of patients who developed at least one grade 3 or 4 haematologic toxicity of any type; 88% versus 14%, respectively). The second most commonly reported acute toxicity was grade 3 to 4 GI toxicity, which was reported in 20% versus 13% of patients in the AP and WAI arms, respectively, and hepatic toxicity was reported in 1% versus 3% of patients in AP and WAI arms, respectively. Grade 3 to 4 cardiac and neurologic toxicities were seen in 15% and 7% of patients, respectively, receiving AP compared with 0% and less than 1% of patients, respectively, receiving WAI. Other infrequent grade 3 to 4 toxicities included hip osteonecrosis, vaginal vault necrosis, and dehydration (one patient each) in the WAI arm and ventral hernia (one patient) in the AP arm Quality of life was reported separately using the Fatigue Scale (FS), Assessment of Peripheral Neuropathy (APN), Functional Alterations due to Changes in Elimination (FACE), and Functional Assessment of Cancer Therapy-General (FACT-G), QoL measured at: pre-treatment, end of treatment (EOT), and 3 and 6 months post-treatment. Of 396 eligible patients, 317 provided a baseline QoL assessment. The AP arm produced a statistically significant survival benefit along with greater toxicities, including peripheral neuropathy persisting up to 6 months. WAI patients reported worse FS (P < 0.001) and FACE (P < 0.001) scores at EOT and poorer FACE scores 3 months post-treatment (P = 0.004) compared to AP patients. APN scores were significantly worse among AP patients at EOT, and 3 and 6 months post-treatment (P < 0.001 for all). There is no indication that FACT-G scores differed between the two arms at any assessment point |
|
| Notes | Treatment probably contributed to eight deaths on the AP arm (two patients had sepsis, two patients had congestive heart failure, and one patient each had sepsis plus left ventricular/aortic thrombus, hypoglycemic shock with myelosuppression, stroke secondary to congestive heart failure, and renal failure with severe thrombocytopenia) and five deaths on the WAI arm (one patient each had veno-occlusive liver disease, disease progression with hepatomegaly, aspiration and liver necrosis, renal and liver failure secondary to sepsis with severe ascites, and sepsis and liver failure) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The GOG Statistical and Data Center randomly assigned therapy to each patient with equal probability of assignment to each treatment regimen. A balanced block randomisation was used to balance assigned treatment regimens within each institution |
| Allocation concealment (selection bias) | Low risk | The sequence of treatment assignments was concealed from institutions and patients until telephone registration with verification of eligibility |
| Blinding (performance bias and detection bias) All outcomes |
High risk | The trial was supervised by the GOG Data Monitoring Committee without knowledge of treatment results. However, treatment allocation could not be concealed for the trial participants |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for the exclusion of 34 patients (15 on the WAI arm and 19 on the AP arm) included wrong stage (n = 3), double primary (n = 8), wrong cell type (n = 4), prior malignancy (n = 1), residual disease more than 2 cm (n = 1), incomplete lymph node sampling or laparoscopic surgery (n = 8), registration error (n = 1), and inadequate documentation of pathology (n = 8). The eight patients (four on each arm) deemed ineligible because of unilateral lymph node sampling or use of laparoscopic surgery are included in the analyses. Since this study was initiated, acceptance and use of laparoscopic surgery has widened, and these patients are otherwise eligible. Their inclusion does not change the study results |
| Selective reporting (reporting bias) | High risk | It was suggested at the ASCO annual meeting by Thomas 2003 that the data were originally presented with a focus on survival time when mortality reached 50% exaggerating the real differences between the two groups The GOG 122 data were reported to show a survival advantage to adjuvant chemotherapy over wide field whole abdominal external beam radiotherapy. Both at the initial data presentation and final publication two interpretations of the data were given. The data as randomised gave five-year outcomes: PFS raw RT 38% versus chemo 42% (P not given), stage-adjusted 38% versus 50% (SS); raw OS RT 42% versus chemo 53% (P not given), stage-adjusted (despite it being randomised trial) 42% versus 52% (SS). Since the rate of recurrence between the two arms differed by only 4% (54% versus 50%) it is unclear whether the differences are due to action on cancer or confounding effects |
| Other bias | High risk | The trial took 10 years to complete from 1992. As a consequence, the inclusion criteria were very broad. The study population included women with an exceptionally good prognosis after hysterectomy, others has an exceptionally bad prognosis. The adjuvant therapies are not optimal by current standards. A dose of 30 g in 20 fraction has only a 10% chance of eradicating upper abdominal disease. The survival curves converge and the benefit for chemotherapy is early and applies only to distant metastases. There was no stratification for risk, particularly site and volume of residuum and we do not know how well balanced the groups were with respect to specific risk factors. These criticisms were eloquently delivered by Thomas 2003 in a plenary session discussion available on the ASCO media player The authors of the definitive manuscript (GOG 122) say that the treatment arms were balanced in terms of patient characteristics but there are slight imbalances with respect to mixed cell type and FIGO stage. If anything, there were slightly more poor prognosis pathologies and more advanced stage cancer in the group allocated chemotherapy |
| Main problems with the trial as perceived by this analysis | High risk | This is predominantly a trial of advanced disease. Few participants had stage 1 or 2 cancer and the data may not be applicable to early disease The applicability of the “control” arm remains uncertain as this trial tests whole abdominal radiotherapy. The radiotherapy regimen used in GOG-122 was tested in only two prospective phase 2 studies, one of which reported after GOG-122 had completed randomisation. The first was GOG 9001 with ten women having whole abdominal radiotherapy with concomitant chemotherapy for resected stage pT3 to pT4 disease in 1990-1992. The second was GOG 94 and this is a description of 21 women with stage pT1 to pT4 endometrial cancer. In 2010, whole abdominal radiotherapy in remains outside the standard treatments suggested by major guideline groups (IOG (UK), National Cancer Institute, USA, National Comprehensive Cancer Network, Lukka 2006). These two factors may explain why the study did not change standard practice. Nevertheless this study fulfils the search criteria for the inclusion in this meta-analysis using the original unadjusted data |
| Methods | This randomised study was designed to assess whether adjuvant chemotherapy confers an advantage for overall and progression-free survival and on the incidence of local and distant relapses over standard pelvic radiotherapy in high-risk patients without residual tumour | |
| Participants | A total of 491 women with high-risk endometrial carcinoma were consecutively referred to 29 institutions throughout Italy. A total of 345 patients were deemed eligible for this study, with 168 randomly assigned external RT and 177 adjuvant CT Women had histologically confirmed endometrioid, adenoacanthoma or adenosquamous carcinoma and FIGO stage Ic G3, IIa-bG3 with deep myometrial invasion (50% or more) or stage III disease following hysterectomy and bilateral salpingo-oopherectomy. To rule out FIGO stage IV disease all patients underwent chest radiography and abdominal-pelvic ultrasound. Women had to have had surgery as primary treatment and no previous neoadjuvant therapy Approximately 70% of the patients had myometrial invasion deeper than 50%. About one third of the patients had FIGO stage I-II disease. 8% had stage 2b cancer. Two thirds stage 3.22% in each group had stage 3c disease and about 40% had stage 3a disease |
|
| Interventions | Women were randomly allocated chemotherapy or radiotherapy after surgical staging and histological evaluation. Chemotherapy had to start within 30 days from surgery. Cyclophosphamide 600mg/m2, adriamycin (doxorubicin) 45mg/m2 and cis-platin 50mgm2 (CAP regimen) were administered every 28 days for five cycles Radiotherapy had to start within 30 days after surgery. External radiation therapy was adopted for a total of 45 to 50 Gray in 5 to 7 weeks (1.7-2 Gray/day, 5 days/week). The upper limit of the pelvic field was at L5, the lower limit at the lower limit of the ischial tuberosity, and the lateral limits fell behind the border of the lateral and common iliac lymph nodes. Patients who had lymph node involvement received additional lumboaortic lymph node irradiation with the upper limit at LI, with 45 Gray in 5 to 7 weeks (1,5-1.8 Gray/day × 5days/ week) |
|
| Outcomes | The primary end points for this trial were overall survival, defined as the time from randomisation to death, irrespective of the cause, and progression-free survival, defined as the time from randomisation to the earliest tumour relapse, or death | |
| Results (OS & PFS) | The hazard ratio (HR) for death after a median follow-up of 95.5 months for women in the chemotherapy group as compared with the radiotherapy group was 0.95 (95% confidence interval (CI), 0.66 to 1.36; P = 0.77). The HR for an event was 0.88 (95% CI, 0.63 to 1.23; P = 0.45). The 3, 5 and 7-year overall survivals were 76%, 66% and 62% in the chemotherapy group 78%, 69% and 62% in the radiotherapy group. The 3, 5 and 7-year progression-free survivals were, 68%, 63% and 60% and 69%, 63% and 56% respectively At the median follow-up time of 95.5 months (interquartile range 62 to 122 months), 135 events (recurrences or deaths, whichever came first) had occurred among the 340 randomised patients. There were 56 recurrences and 10 deaths as first event of the 174 patients on chemotherapy and 60 recurrences and nine deaths as first event of the 166 patients on radiotherapy. The overall number of observed deaths was 118 (35%). Fifty-nine in the RT arm and 59 in the CT arm The overall survival of the patients on chemotherapy was 76% (CI = 70% to 83%), 66% (CI = 59% to 73%) and 62% (CI = 55% to 70%) at the third, fifth and seventh year, respectively, and 78% (CI = 71% to 84%), 69% (CI = 61% to 76%) and 62% (CI = 54% to 71%) for patients on radiotherapy at the same time points. The progression-free survival of the patients on chemotherapy was 68% (CI = 61% to 75%), 63% (CI = 55% to 70%) and 60% (01 = 52% to 67%) at the third, fifth and seventh year, respectively and 69% (CI = 62% to 77%), 63% (CI = 55% to 70%) and 56% (CI = 46% to 63%) for patients on radiotherapy |
|
| Results (site of recurrence) | Chemotherapy delayed distant metastases and radiotherapy delayed local relapses but these trends did not achieve statistical significance. Among the 174 patients randomised to chemotherapy, the initial site of recurrence was distant in 27 (16%), local in 19 (11%), concurrent local and distant in eight (5%), and of unknown type in two (1%). Among the 166 patients randomised to radiotherapy the initial site of recurrence was distant (extra-abdominal or liver) in 35 (21%), local in 11 (7%), concurrent distant and local in nine (5%), and of unknown type in five (3%). Although this study was not powered to detect clinically significant differences in the incidence of relapses, chemotherapy seemed to prevent or delay distant relapses more than radiotherapy while radiotherapy seemed to prevent or delay local relapses in comparison with chemotherapy | |
| Toxicity | Full details about the toxicity of chemotherapy with CAP is available for 123 women (80% of the 154 patients who had at least one course). Grades 2, 3 and 4 neutropenia occurred in 22 (18%), 38 (31%) and 5 (4%) patients, respectively; 36 patients (29%) had grade 2 anaemia, 5 (4%) had grade 3 anaemia; grade 2 and 3 thrombocytopenia was reported in five (4%) and two patients (2%), respectively. The incidence of nausea and vomiting was relatively low (grade 2 and 3 was reported for 29 (24%) and 12 (10%) patients, respectively, grade 4 for one patient). Other serious toxicities (grade 3) occurred in < 3 % of the patients randomised to CT. There were no treatment-related deaths Toxicity data are available for 146 (97%) of the 150 patients who started radiotherapy. Major late toxic effects were gastrointestinal, including five cases of bowel obstruction with three of these patients requiring surgical intervention, six cases of grade 3 radiation proctitis, and 13 reports of grade 3 diarrhoea (24 patients, 16%). Urinary tract complications (severe cystitis) were recorded in seven patients (5%) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out centrally by telephone at the L Mangiagalli Institute (Milan, Italy) and patients were stratified by institution and stage of disease |
| Allocation concealment (selection bias) | Low risk | It is very unlikely that there was any bias or allocation concealment during analysis of data but this is not clear in the reports |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It is not possible to conceal the treatment allocation from the participants |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 131 (75%) of the 174 women assigned chemotherapy received five treatment cycles as planned and 154 (89%) received at least one cycle and were assessable for toxicity (six, four, four and nine women received only one, two, three and four courses of CAP, respectively, mainly because of excessive bone marrow toxicity). 12 women (7%) declined adjuvant chemotherapy 146 (88%) of the 166 women assigned radiotherapy completed treatment as planned. Only four women (2%) stopped treatment because of toxicity. Ten women (6%) declined treatment |
| Selective reporting (reporting bias) | Low risk | The trial is reported according to CONSORT criteria and there is no suggestion of any reporting bias |
| Other bias | Low risk | The two groups were similar across all categories. |
| Main problems with the trial as perceived by this analysis | Low risk | Threre are no serious flaws in this trial. |
| Methods | This trial utilised a straightforward randomisation comparing either pelvic irradiation or chemotherapy after initial surgical excision | |
| Participants | A total of 475 women were randomised. Forty-one were deemed to be ineligible due to protocol violations such as myometrial invasion was less then 50% of the way through the myometrium, histology review revealed a sarcoma or rapid progression after enrolment. An additional 49 cases were excluded from analysis due to non-endometroid histology. The eligibility criteria for this study were International Federation of Gynecology and Obstetrics (FIGO- prior to 1998) stage IC-IIIC endometrial carcinoma with deeper than 50% myometrial invasion and absence of any prior chemotherapy, irradiation, or surgery for the treatment of any other cancer. Patients with stage II or III without deeper than 50% myometrial invasion were ineligible for this study. Patients were required to be under 75 years old, to have a WHO performance status of 0 to 3, and to have no residual visible tumour after an abdominal hysterectomy and bilateral salpingo-oophorectomy. Surgical staging consisted ideally of pelvic and/or para-aortic lymphadenectomy. 96% of women had a pelvic lymphadenectomy, 29% had a para-aortic lymphadenectomy | |
| Interventions | The chemotherapy group received cyclophosphamide (333 mg/m2), doxorubicin (40 mg/m2), and cisplatin (50mg/m2) (CAP chemotherapy) every 4 weeks for 3 or more courses. Dose modifications of doxorubicin and cisplatin were as follows: a 25% reduction of both drugs was allowed for body weight less than 40 kg or age greater than 70 years old, and a 50% reduction was allowed in patients with G3 or G4 myelosuppression Pelvic irradiation was given in an open field using the anterio-posterior parallel opposing technique within 4 weeks of surgery. Forty-five to fifty Gray was given within 4 to 6 weeks, with 9 to 10 Gray of irradiation administered per week (5 working days per week). Subsequently, additional irradiations were performed in 11 cases (5.7%) with paraaortic lesions and in 6 patients (3.1%) who received brachytherapy | |
| Outcomes | The primary endpoint was OS and secondary endpoints were PFS and the incidence of toxicity | |
| Results (OS & PFS) | The PFS rate at 5 years was 83.5% in the PRT group and 81.8% in the CAP group. The hazard ratio was 1.07 (95% CI, 0.65 to 1.76:P = 0.73) The OS rate at 5 years was 85.3% in the PRT group and 86.7% in the CAP group. The hazard ratio was 0.72 (95% CI, 0.40 to 1.29; P = 0.27) |
|
| Results (site of recurrence) | Thirty-three recurrences (17%) occurred in the chemotherapy group compared to 30 recurrences (15%) in the radiotherapy group. The patterns of recurrence were similar in both treatment groups. Specifically, the incidence of intrapelvic recurrence sites, such as the pelvis or vagina, was 7.3% (14/192) in the chemotherapy group and 6.7% (13/193) in the radiotherapy group while the incidence of extrapelvic recurrence sites, such as the peritoneal cavity, liver, lung, para-aortic lymph nodes and others, was 16.1% (31/192) and 13.5% (26/193) respectively | |
| Toxicity | G3 and G4 toxicities were experienced in 4.7% (9/192) of women given chemotherapy and 1.6% (3/193) of women allocated radiotherapy. Myelosuppression was the main problem for women given chemotherapy and bowel obstructions were the main complication in the radiotherapy group No treatment-related deaths occurred in either group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation involved simple randomisation. Each participant was assigned by central telephone system The study groups were remarkably well balanced for patient characteristics including age, postmenopausal status, co-morbidity, type of hysterectomy, postoperative stage, tumour grade, myometrial invasion, lymphovascular space invasion, cervical involvement, parametrial invasion, peritoneal cytology, adnexal metastasis, pelvic lymph node metastasis, and para-aortic lymph node metastasis. Treatment was completed in 98.9% (184/186) and 97.3% (183/188) of the patients in the PRT and CAP groups, respectively |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It is not possible to conceal the treatment allocation from the participants |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | The initial enrolment involved 475 women. Forty-one were ineligible due to myometrial invasion of less than 50%, histological diagnosis of sarcoma, or rapid progression of disease after enrolment. An additional 49 patients with non-endometrioid histology were excluded. As a result, 385 patients were eligible for this trial. Seven patients in the PRT group did not receive PRT and 4 patients in the CAP group did not receive CAP Treatment was completed in 98.9% (184/186) and 97.3% (183/188) of the patients in the radiotherapy and chemotherapy groups, respectively. Pelvic radiation was defined as completed when the total radiation dose reached 40 Gray. Chemotherapy was said to have been completed when the number of cycles reached three. The median total doses were 50 Gray of pelvic irradiation and 1309 mg/m2 cyclophosphamide, 120 mg/m2 doxorubicin, and 180 mg/m2 cisplatin. The median number of chemotherapy courses was 3, ranging from 1 to 7. The median duration of treatment was 5.1 weeks and 11.4 weeks in the radiotherapy and chemotherapy groups, respectively |
| Selective reporting (reporting bias) | High risk | The overall trial showed no significant difference with five-year PFS 83% versus 82%; OS 85% versus 87%. A subgroup analysis of 120 women in a high- to intermediate-risk group defined as (1) stage pTIc over 70 years old or with grade3 endometrioid adenocarcinoma or (2) stage pT2 or pT3a (positive cytology) was presented in the same publication. It suggested the chemotherapy group had a significantly higher PFS rate (83.8% versus 66.2%, log-rank test P = 0.024, hazard ratio 0.44) and higher OS rate (89.7% versus 73.6%, log-rank test P = 0.006, hazard ratio 0.24). Nevertheless this study fulfils the search criteria for the inclusion in this meta-analysis using the original unselected data |
| Other bias | Low risk | The broad inclusion criteria and subgroup analysis has been criticised but the authors have never deviated from their initial protocol and the groups are remarkably well matched However, the graphs depicting overall survival describe 122 women allocated radiotherapy but the graph related to PFS provides data on 123 women |
| Main problems with the trial as perceived by this analysis | High risk | Other trials have used higher doses of chemotherapy and used a 3 weekly cycle, not 4 weekly The other issue worth of note is the inconsistent data reporting. In the early ASCO presentations of this trial , 208 women were described as having low or intermediate risk disease and 67 women had high-risk disease. The full report published in 2008 has slightly different numbers. According to the manuscript, 190 women had low or intermediate risk disease, 120 had high intermediate risk group and 75 had high-risk disease Low intermediate risk was defined in the manuscript and as deeply invasive, well or moderately differentiated cancer. Of the 190 women reported in them anuscript, the progression-free survival was 94.5% and 87.5% (P = 0.11) and overall 5 year survival was 95% and 91% (P = .2) associated with radiotherapy and chemotherapy respectively. Earlier presentations described 89.6% and 87.2% The high intermediate risk group had deeply invasive poorly differentiated tumours and involved women over the age of 70 or they had stage 2 or 3a cancer. There were 120 women in the manuscript and there was significant progression-free survival advantage associated with chemotherapy HR = .44 (.20 to .97) High-risk disease was defined as stage 2 and 3a in the ASCO presentation of 67 women and they gained a substantial progression-free survival advantage (64.2% versus 84.5%; P = 0.06) and an overall survival benefit from adjuvant chemotherapy (80.3% versus 97.5% P = 0.02). This benefit was not confirmed in the final manuscript. In fact, the manuscript describes a progression-free survival rate in this group at 5 years of 78.6% and 64.4% (P = 0.17) and the overall survival rate at 5 years of 75.7% and 71.1% respectively. This is now a non significant trend in favour of radiotherapy. Presumably the data matured with time as more events were reached |
| Methods | GOG orchestrated multicenter phase 3 randomised controlled trial | |
| Participants | A total of 206 women with any stage of previously untreated locally confined mixed mesenchymal mullerian malignant tumour (primary carcinosarcomas of the uterus) were randomised to whole abdominal irradiation (WAI) arm (n = 105) or chemotherapy (n = 101) with Cispltain, Ifosfamide and mesna All women had a hysterectomy, bilateral salpingo-oopherectomy and optimal resection of all gross intra-abdominal/pelvic disease, including macroscopically involved pelvic and para-aortic nodes, leaving no residual disease any larger than 1 cm before randomisation Twenty-five cases were excluded from the analysis based on review of histology and one was excluded because the GOG committee felt there was inappropriate residual disease at randomisation |
|
| Interventions | Chemotherapy consisted of intravenous (IV) cisplatin (20mg/m2/day × 4 days) followed by a 1 hour infusion of ifosfamide (1.5g/m2/day IV × 4 days) with mesna (120 mg/m2 IV bolus over 15 min on day one, followed by 1.5 g/m2/day IV continuous infusion × 4 days beginning with day one) every 3 weeks for three cycles within 8 weeks following initial surgery Whole abdominal irradiation involved 30 Gray to the whole abdomen at 1 Gray per fraction, two fractions per day, and 5 days each week with a minimum of 6 hour between morning and afternoon fractions (hyperfractionation). Due to slow patient accrual, in August 1996, the dose fractionation schedule was changed to once-daily fractions of 1.5 Gray for 5 days each week to the same total dose to the whole abdomen of 30 Gray. Early recruits were given a pelvic boost of hyperfractionated 20 Gray. The fractionation schedule was also changed in August 1996 to once-daily fractions of 1.8 Gray for 5 days each week to a total dose of 19.8 Gray (cumulative true pelvic dose of 49.8 Gray) |
|
| Outcomes | Overall survival was defined as the time from randomisation to death from any cause or, for living patients, the date of last contact. The recurrence-free interval was assessed from the date of randomisation to the date when clinically evident disease was observed | |
| Results (OS & PFS) | The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). Adjusting for stage and age, the recurrence rate was 21% lower for CIM patients than for WAI patients (relative hazard (RH) = 0.789, 95% confidence interval (CI): (0.530 to 1.176), P = 0.245, 2-tail test). The estimated death rate was 29% lower among the CIM group (RH = 0.712, 95% CI: 0.484 to 1.048, P = 0.085, two-tail test) The estimated crude probability of surviving at least five years following diagnosis was approximately 35% for those randomised to WAI versus 45% for those randomised to CIM. After adjusting for stage and age at diagnosis, the estimated death rate was 29% lower for CIM patients than WAI patients (RH = 0.712, 95% CI: 0.484 to 1.048). A similar result was obtained when all women (regardless of eligibility) were included in the analysis of survival for an intention-to-treat analysis (RH = 0.727, 95% CI: 0.503 to 1.050) |
|
| Results (site of recurrence) | There have been 112 recurrences reported from 206 women. These include 60 in the radiotherapy group and 52 in the chemotherapy group. There were slightly more vaginal recurrences in the CIM group and more abdominal recurrences in the WAI group, but these differences were not statistically significant The sites of first recurrence for women allocated radiotherapy included the vagina (4/105; 3.8%), pelvis (14/105; 13.3%), abdomen (29/105; 27.6%) and distant sites 13/105; 25.7%). The corresponding sites of first recurrence for women allocated chemotherapy was the vagina (10/101; 9.9%), pelvis (14/101; 13.8%), abdomen (19/101; 18.8%) and other distant sites (10/101; 23.8%) |
|
| Toxicity | Grade 3 or 4 acute anaemia (11 versus 1, P < 0.01) and neuropathy (central and peripheral) (9 versus 0, P < 0.01) occurred more often among those treated with CIM chemotherapy than radiotherapy. Regarding grade 2, 3 or 4 late effects, gastrointestinal events occurred more often among those treated with radiotherapy (WAI) than chemotherapy (10 versus 0, P < 0.001) Two women in the radiotherapy cohort died as a direct consequence of radiation hepatitis, while one patient in the chemotherapy arm died of an acute systemic blood infection which originated at the port site and was complicated by neutropenia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was orchestrated by the central GOG office block randomisation based |
| Allocation concealment (selection bias) | Low risk | The authors confirm that treatment assignments remained concealed from randomisation code generation to registration |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It was not possible to blind the observers or participants to treatment allocation in this type of trial |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Primary outcome data were available on all 206 women and data on adverse events was available from 197/206 (96%) cases. A total of 232 women were randomised, but 26 were found to be ineligible and excluded (appropriately before analysis |
| Selective reporting (reporting bias) | Low risk | There is no suggestion of selective reporting and all important survival events have been reported |
| Other bias | Low risk | We have been unable to find any suggestion from any author that the study was biased |
| Main problems with the trial as perceived by this analysis | Low risk | The protocol for delivering radiotherapy changed midway through the study but this is unlikely to have any impact on the conclusions that can be derived from this study |
| Methods | see table for the separate studies |
| Participants | |
| Interventions | |
| Outcomes | |
| Results (OS & PFS) | |
| Results (site of recurrence) | |
| Toxicity | |
| Notes |
Characteristics of excluded studies [author-defined order]
| Study | Reason for exclusion |
|---|---|
| Bruner | 2007 This report describes quality of life in the GOG 122 trial. These data were included in the review but the manuscript has no data on the primary outcomes |
| Brunetto 2000 | This was a trial examining the role of ifosfamide for uterine tumours but the morphology was not a simple adneocarinoma. The study population had advanced, persistent or recurrent carcinosarcoma of the uterus. Consequently the data were excluded as they do not address the primary management of a women presenting with a new cancer: |
| Deng 2000 | This trial examined the effect of radiotherapy and chemotherapy on uterine morphology. It is not included in the analyses because there are no outcome data This study reported on the histological changes in 58 women treated for endometrial carcinoma with Estramustine phosphate (EMP) or radiotherapy. Estramustine (Emcyt, Estracit) is a chemotherapy agent commonly used to treat prostate cancer derived from estradiol with a nitrogen mustard-carbamate ester moiety that makes it an alkylating antineoplastic agent similar to mechlorethamine, with oestrogen-induced specificity. Women were randomly divided into three groups and clinically observed. Twenty-one women allocated oral Estramustine had 280 mg twice daily for 21 days before surgery and 19 women allocated radiotherapy received preoperative intra-cavity irradiation with half of the standard dosage. The control group involved 18 women who received surgical operation alone. The microscopic changes induced by irradiation were much heavier than those induced by chemotherapy. 5/21 women given Estramustine were found with no tumour lesion in the post operation samples, all of those five cases had strongly positive oestrogen receptors (++) and four of the five cases were well differentiated tumours before chemotherapy. In radiotherapy group, the tumour lesion disappeared in 6/19 cases, and five were moderately differentiated. No histopathological changes were seen in control group. Immunohistochemical tests revealed a significant decrease in oestrogen receptor staining after Estramustine and a decrease in ki- 67 index, especially for the oestrogen receptor positive tumours. The ki-67 index reduced significantly from 49.5% before to 35.1% after medication (P < 0.05). |
| Chauvergne 2008 | This study compared different chemotherapy regimens but was excluded as the treatment allocation did not use a random allocation program |
| GCSF | The study population had advanced endometrial carcinoma, and the trial did not focus on the primary treatment after hysterectomy |
| GOG 107 | This phase III trial examined the addition of doxorubicin to cisplatin in advanced endometrial carcinoma |
| GOG 156 | The trial was never completed due to poor accrual |
| GOG 194 | The trial was never completed due to poor accrual |
| Fujimura 2000 | This study compared different chemotherapy regimens but is excluded as the treatment allocation did not involve a group that was not given chemotherapy The trial compared the outcome after randomly allocating women with deeply invasive (old stage 1C) endometrial endometrioid adenocarcinoma after hysterectomy either adjuvant CAP (cyclophosphamide, pirarubicin and cisplatin) or EP (etoposide and cisplatin). The five-year survival rate was 88.4% in the CAP group and 95.1% in the EP group; the difference between the two groups was not significant (P = 0.3496). The disease-free survival rate was 80.3% in the CAP group and 84.8% in the EP group (nonsignificant: P = 0.4533) |
| Omura | This was a study of women with stage I and II uterine sarcoma who were randomly allocated Doxorubicin (adriamycin) or no adjuvant chemotherapy. Participants did not have endometrial cancer |
| Samuels 2004 | This was a randomised study of trabectedin given by different dosing schedules in patients with uterine leiomyosarcomas or liposarcomas, not cancers |
| Sutton 2000 | This was a randomised trial comparing ifosfamide with ifosfamide + cisplatin involving 194 women with advanced or recurrent carcinosarcoma no longer amenable to control by surgery or radiotherapy |
| GOG 161 | Phase III trial studied the addition of ifosfamide to paclitaxel in advanced uterine carcinosarcoma |
| Aapro 2003 | This study recruited women with advanced inoperable or recurrent endometrial cancer and they were randomly assigned doxorubicin alone or in combination with cisplatin |
| Cohen 1984 | This study recruited women with advanced, recurrent or residual endometrial adenocarcinoma considered incurable by radiation or surgery. Women were randomised to melphalan, 5-fluorouracil and megace or doxorubicin, cyclophosphamide and 5-fluorouracil |
| Edmonson 1987 | This study recruited women with advanced cancer. Women with progestin-refractory, metastatic endometrial cancer were randomised to receive cisplatin alone or cisplatin in combination with doxorubicin and cyclophosphamide |
| Fleming 2004a | This study recruited women with advanced (stage III, IV) or recurrent endometrial cancer. They were randomised to receive doxorubicin and cisplatin or doxorubicin, cisplatin and paclitaxel with Granulocyte colony stimulating factor support |
| Fleming 2004b | This study recruited women with primary stage III, stage IV or recurrent endometrial carcinoma. They were randomised to receive doxorubicin and cisplatin or doxorubicin, paclitaxel and G-CSF support |
| Gallion 2003 | This study recruited women with primary stage II, IV or recurrent endometrial carcinoma. They were randomised to standard-timed doxorubicin or circadian-timed doxorubicin and cisplatin |
| Horton 1978 | This small study recruited women with measurable local extension or metastases not amenable to cure by surgery or radiotherapy. The women were randomised to receive doxorubicin or cyclophosphamide |
| Long 1995 | This small study described in abstract form recruited women with advanced cancer. They were randomised to receive doxorubicin and cisplatin or doxorubicin, cisplatin, methotrexate and vinblastine |
| Pawinski 1999 | This study recruited women with recurrent or metastatic disease. They were randomised to receive cyclophosphamide or ifosfamide |
| Thigpen 1994 | This study recruited women with advanced cancer. Women had advanced or recurrent endometrial carcinoma no longer amenable to surgery or radiotherapy and were randomised to receive doxorubicin alone or in combination with cyclophosphamide |
| Thigpen 2004 | This study recruited women with advanced cancer or recurrent endometrial carcinoma. They were randomised to treatment with doxorubicin alone or in combination with cisplatin |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Phase III Trial of pelvic radiation therapy versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy |
| Methods | Randomisation to either pelvic radiotherapy or vaginal brachytherapy followed by 3 cycles carboplatin/paclitaxel |
| Participants | Women with high intermediate and high-risk stage I-II endometrial carcinoma |
| Interventions | Arm I: Active Comparator Patients undergo conventional or intensity-modulated pelvic radiotherapy once daily, 5 days a week, for 5 to 6 weeks (total of 25 to 28 fractions) in the absence of disease progression or unacceptable toxicity. Patients with stage II disease or stage I disease with a confirmed diagnosis of clear cell and/or papillary serous histology may also undergo 1 or 2 intravaginal (i.e., vaginal cuff) brachytherapy boost treatments Arm II: Experimental Patients undergo vaginal cuff brachytherapy comprising 3 to 5 high-dose rate brachytherapy treatments over approximately 2 weeks or 1 or 2 low-dose rate brachytherapy treatments over 1 to 2 days. Beginning within 3 weeks after initiating brachytherapy, patients receive paclitaxel IV over 3 hours and carboplatin IV over 30 to 60 minutes on day 1. Chemotherapy repeats every 21 days for up to 3 courses in the absence of disease progression or unacceptable toxicity |
| Outcomes | Primary Outcome Measures: Duration of recurrence-free survival Secondary Outcome Measures:
|
| Starting date | March 2009 |
| Contact information | Study Chair: D. Scott McMeekin, MD Oklahoma University Cancer Institute; Phone +1 405-271-8707 Investigator: Marcus E. Randall and Lucille P. Markey, Cancer Center at University of Kentucky, phone +1 859-257-7618 |
| Notes |
| Trial name or title | A Randomized Phase III Trial of Cisplatin and Tumor Volume Directed Irradiation Followed by Carboplatin and Paclitaxel vs. Carboplatin and Paclitaxel for Optimally Debulked, Advanced Endometrial Carcinoma |
| Methods | Randomisation to either volume-directed radiotherapy with complex platinum based chemotherpay or 6 cycles conventional dose Carboplatin plus Paclitaxel. Additionally, tissue is stored for translational research |
| Participants | All women have surgical stage III or IVA endometrial carcinoma per FIGO 1988 staging criteria of any histological type. Surgical Stage III disease includes tumour invading the serosa, tumour in the adnexa, pelvic and/or para-aortic nodes, or vaginal involvement. Women with tumour cells in the pelvic washings as the only extra-uterine disease are eligible only if the histology is clear cell or serous papillary. Surgical Stage IVA includes patients with bladder or bowel mucosal involvement, but no spread outside the pelvis. Interim analysis in January 2011 shows that initial data was available from 61 women and all had stage 3 disease Partisipants have a GOG Performance Status of 0, 1, or 2 and are 18 years of age or older. Entry into the study is limited to no more than 8 weeks from the date of surgery. Surgery involves a hysterectomy and bilateral salpingo-oophorectomy. Pelvic lymph node sampling and para-aortic lymph node sampling are optional The volume of residual disease after surgery must be less than 2cm in any dimension |
| Interventions | Participants are randomly allocated to either
|
| Outcomes | To determine if treatment with cisplatin and volume-directed radiation followed by carboplatin and paclitaxel for 4 cycles (experimental arm) reduces the rate of recurrence or death (i.e. increases recurrence-free survival) when compared to chemotherapy consisting of carboplatin and paclitaxel for 6 cycles (control arm) |
| Starting date | The study opened to recruitment on June 29, 2009 |
| Contact information | The study chairs for the GOG include Daniela Matei, Marcus E. Randall, David Mutch, Ursula A. Matulonis, Michael J. Birrer and Margaret Steinhoff. I-Chow Hsu represents the RTOG |
| Notes | The target recruitment is 804. By July 2010, 69 women had been recruited |
| Trial name or title | A Phase III intergroup trial on adjuvant therapy in radically operated endometrial cancer patients with high risk for micro-metastatic disease: 4 courses of adjuvant carboplatin/paclitaxel followed by radiation therapy versus 2 more courses of carboplatin/paclitaxel |
| Methods | Women are treated according to randomisation to either adjuvant radiotherapy (48.6 Gray in 1.8 Gray fractions) + para-aortic irradiation if pelvic N+ and para-aortal nodes NX or N+ or 2 additional cycles of adjuvant CT (carboplatin and paclitaxel) The randomisation occurs after surgery and before the routine adjuvant chemotherapy. Vaginal brachytherapy is added if stage II or higher with cervical engagement if extended hysterectomy with parametrial resection has not been performed |
| Participants | Women treated by hysterectomy, four courses of chemotherapy (carboplatin and paclitaxel) for histologically confirmed endometrial carcinoma (endometrioid, adenosquamous, serous or clear cell carcinoma) with no macroscopic remaining tumour after primary surgery (lymph node exploration optional), with one of the following postoperative FIGO 2009 stage and grade:
WHO-performance status 0-2; |
| Interventions | After randomisation all patients receive four courses of CT (carboplatin and paclitaxel). Thereafter women are treated according to randomisation Arm 1: Adjuvant radiotherapy (48.6 Gray in 1.8 Gray fractions) + para-aortic irradiation if pelvic N+ and para-aortal nodes NX or N+ Arm 2: 2 additional cycles of adjuvant CT (carboplatin and paclitaxel) Vaginal brachytherapy is added if stage II or higher with cervical engagement if extended hysterectomy with parametrial resection has not been performed |
| Outcomes |
Primary: Overall survival (OS) Secondary: Progression-free survival (PFS), Failure-free survival (FFS; defined as relapse or death from endometrial carcinoma or of treatment complications, deaths of intercurrent causes censored), treatment toxicity, quality of life, translational research of predictive or prognostic factors |
| Starting date | |
| Contact information | Thomas Hogberg, Department of Cancer Epidemiology, University Hospital, SE-221 85 Lund, Sweden. thomas.Hogberg@med.lu.se |
| Notes |
| Trial name or title | Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high-risk and advanced stage endometrial carcinoma: PORTEC-3 |
| Methods | Randomised controlled trial |
| Participants | Women with high-risk and advanced stage endometrial carcinoma
|
| Interventions | Patients are randomised (1:1) to receive external beam pelvic radiotherapy (standard arm: 48.6 Gray in 1.8 Gray fractions), or pelvic radiotherapy with concurrent chemotherapy (two cycles of cisplatin) followed by adjuvant chemotherapy (four cycles of carboplatin and paclitaxel; experimental arm) This trial tests the addition of concurrent and adjuvant chemotherapy to postoperative radiation therapy |
| Outcomes | Five-year overall survival and failure-free survival |
| Starting date | |
| Contact information | Dr C L Creutzberg. c.l.creutzberg@lumc.nl |
| Notes | http://www.clinicalresearch.nl/portec3/ |
DATA AND ANALYSES
Comparison 1.
Comparing survival curves (indiscriminate combination of all RCT hazard ratios)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause (HR) | 6 | Hazard Ratio (Random, 95% CI) | 0.74 [0.62, 0.89] | |
| 1.1 Chemotherpay versus no additional treatment (all receive EBRT+surgery) | 2 | Hazard Ratio (Random, 95% CI) | 0.68 [0.45, 1.02] | |
| 1.2 Chemotherpay v Radiotherapy | 4 | Hazard Ratio (Random, 95% CI) | 0.76 [0.62, 0.92] | |
| 2 Death or recurrence (HR) | 7 | Hazard Ratio (Random, 95% CI) | 0.75 [0.64, 0.89] | |
| 2.1 Chemotherpay versus no additional treatment (all receive EBRT+surgery) | 3 | Hazard Ratio (Random, 95% CI) | 0.63 [0.44, 0.89] | |
| 2.2 Chemotherpay v Radiotherapy | 4 | Hazard Ratio (Random, 95% CI) | 0.80 [0.66, 0.97] |
Comparison 2.
Overall survival 5 years after randomisation (risk of death), all trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of death; Chemotherapy versus any other arm (RR) | 8 | 2197 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.79, 0.99] |
| 1.1 Chemotherpay versus no additional treatment (all receive EBRT+surgery) | 4 | 871 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 1.2 Chemotherapy v radiotherapy | 4 | 1326 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.76, 0.99] |
| 2 Risk difference for death at 5 years for all trials | 8 | 2197 | Risk Difference (IV, Random, 95% CI) | −0.03 [−0.07, 0.00] |
| 2.1 Chemotherpay versus no additional treatment (all receive EBRT+surgery) | 4 | 871 | Risk Difference (IV, Random, 95% CI) | −0.02 [−0.08, 0.03] |
| 2.2 Chemotherapy v radiotherapy | 4 | 1326 | Risk Difference (IV, Random, 95% CI) | −0.04 [−0.10, 0.02] |
| 3 Restricted to trials of conventional chemotherapy (RR) | 6 | 1860 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.76, 0.96] |
| 4 Risk difference restricted to trials of conventional chemotherapy | 6 | 1860 | Risk Difference (IV, Random, 95% CI) | −0.04 [−0.08, −0.01] |
| 5 Analysis excluding unconventional radiotherapy regimens (RR) | 5 | 1439 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.80, 1.16] |
Comparison 3.
Separating the trials; Chemotherapy versus radiotherapy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause; Chemotherapy versus radiotherapy (HR) | 4 | Hazard Ratio (Random, 95% CI) | 0.76 [0.62, 0.92] | |
| 2 Death or recurrence; Chemotherapy versus radiotherapy (HR) | 4 | Hazard Ratio (Random, 95% CI) | 0.80 [0.66, 0.97] | |
| 3 Overall 5 year survival; Chemotherapy versus radiotherapy (RR) | 4 | 1326 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.76, 0.99] |
Comparison 4.
Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause; Chemotherapy versus no additional treatment (HR) | 2 | Risk Ratio (Random, 95% CI) | 0.68 [0.45, 1.02] | |
| 2 Death or recurrence; Chemotherapy versus no additional treatment (HR) | 3 | Hazard Ratio (Random, 95% CI) | 0.63 [0.44, 0.89] | |
| 3 Overall 5 year survival; Chemotherapy versus no additional treatment (RR) | 4 | 871 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
Comparison 5.
Sensitivity analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause (HR) excluding mixed tumours (carcinosarcomas) | 5 | Hazard Ratio (Random, 95% CI) | 0.74 [0.62, 0.89] | |
| 2 Overall 5 year survival (risk of death) excluding mixed tumours (carcinosarcomas) (RR) | 7 | 1991 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.78, 1.02] |
| 3 Overall 5 year survival (risk of death) excluding mixed tumours and trials of unconventional chemotherapy (RR) | 5 | 1654 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.74, 0.98] |
Comparison 6.
Site of first recurrence
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Distant (extrapelvic) recurrence (RR; all trials) | 7 | 2198 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.68, 0.92] |
| 2 Distant (extrapelvic) recurrence; (Risk difference; all trials) | 7 | 2198 | Risk Difference (IV, Random, 95% CI) | −0.05 [−0.08, −0.01] |
| 3 Distant (extrapelvic) recurrence excluding mixed tumours (RR; all trials) | 6 | 1992 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.67, 0.94] |
| 4 Pelvic recurrence (chemotherapy v no treatment) (RR) | 2 | 690 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.20, 1.18] |
| 5 Pelvic recurrence (chemotherapy v radiotherapy) (RR) | 4 | 1327 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.97, 1.68] |
| 6 Any recurrence (chemotherapy v no adjuvant treatment) (RR) | 4 | 869 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.49, 0.84] |
| 7 Any recurrence (chemotherapy v radiotherapy) (RR) | 4 | 1327 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.83, 1.08] |
Comparison 7.
Subgroup analysis of NON-endometriod cancer (clear and serous)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause (HR) for subgroup serous/clear cell | 2 | Hazard Ratio (Random, 95% CI) | 0.98 [0.68, 1.40] | |
| 2 Death or recurrence (HR) for subgroup serous/clear cell cancer | 2 | Hazard Ratio (Random, 95% CI) | 0.84 [0.57, 1.23] |
Analysis 1.1. Comparison 1 Comparing survival curves (indiscriminate combination of all RCT hazard ratios), Outcome 1 Death from any cause (HR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 1 Comparing survival curves (indiscriminate combination of all RCT hazard ratios)
Outcome: 1 Death from any cause (HR)
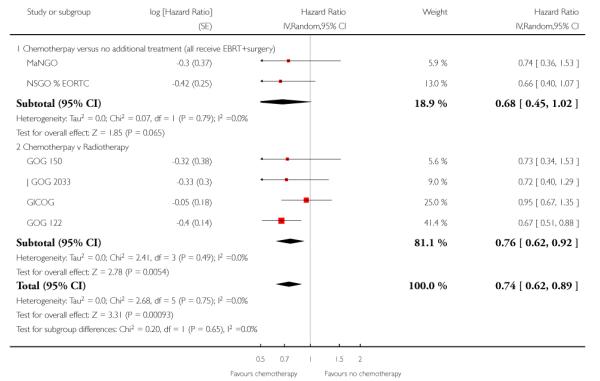 |
Analysis 1.2. Comparison 1 Comparing survival curves (indiscriminate combination of all RCT hazard ratios), Outcome 2 Death or recurrence (HR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 1 Comparing survival curves (indiscriminate combination of all RCT hazard ratios)
Outcome: 2 Death or recurrence (HR)
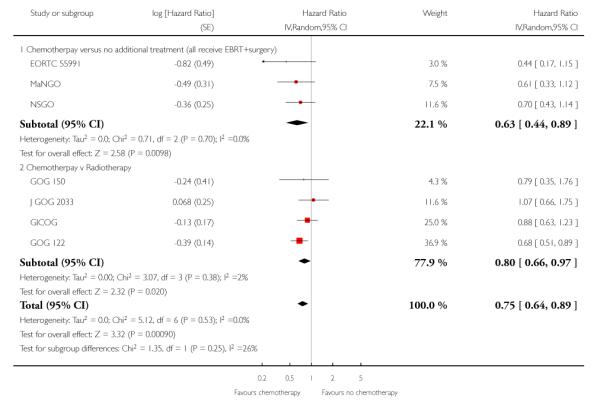 |
Analysis 2.1. Comparison 2 Overall survival 5 years after randomisation (risk of death), all trials, Outcome 1 Risk of death; Chemotherapy versus any other arm (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 2 Overall survival 5 years after randomisation (risk of death), all trials
Outcome: 1 Risk of death; Chemotherapy versus any other arm (RR)
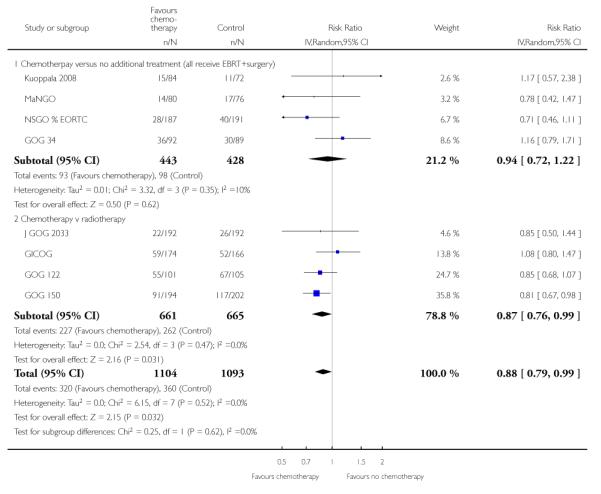 |
Analysis 2.2. Comparison 2 Overall survival 5 years after randomisation (risk of death), all trials, Outcome 2 Risk difference for death at 5 years for all trials
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 2 Overall survival 5 years after randomisation (risk of death), all trials
Outcome: 2 Risk difference for death at 5 years for all trials
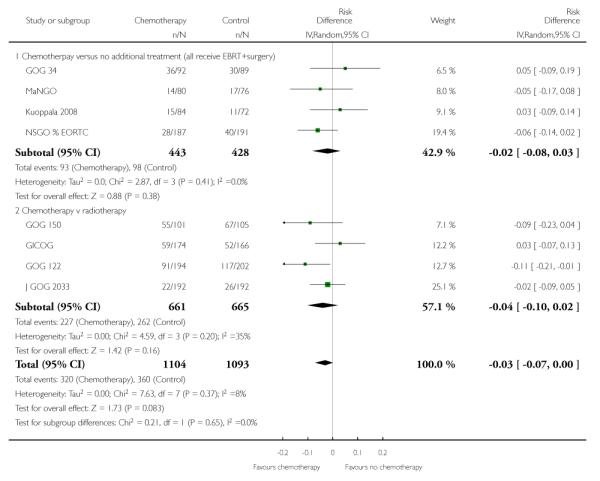 |
Analysis 2.3. Comparison 2 Overall survival 5 years after randomisation (risk of death), all trials, Outcome 3 Restricted to trials of conventional chemotherapy (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 2 Overall survival 5 years after randomisation (risk of death), all trials
Outcome: 3 Restricted to trials of conventional chemotherapy (RR)
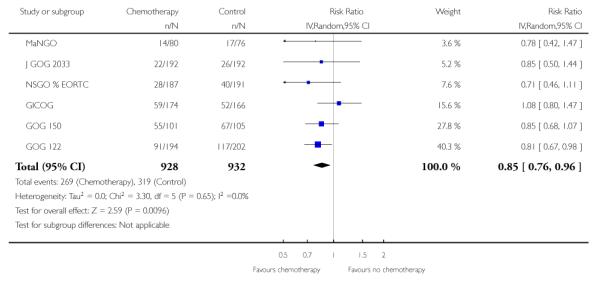 |
Analysis 2.4. Comparison 2 Overall survival 5 years after randomisation (risk of death), all trials, Outcome 4 Risk difference restricted to trials of conventional chemotherapy
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 2 Overall survival 5 years after randomisation (risk of death), all trials
Outcome: 4 Risk difference restricted to trials of conventional chemotherapy
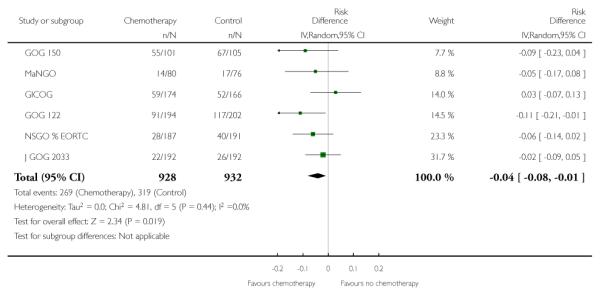 |
Analysis 2.5. Comparison 2 Overall survival 5 years after randomisation (risk of death), all trials, Outcome 5 Analysis excluding unconventional radiotherapy regimens (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 2 Overall survival 5 years after randomisation (risk of death), all trials
Outcome: 5 Analysis excluding unconventional radiotherapy regimens (RR)
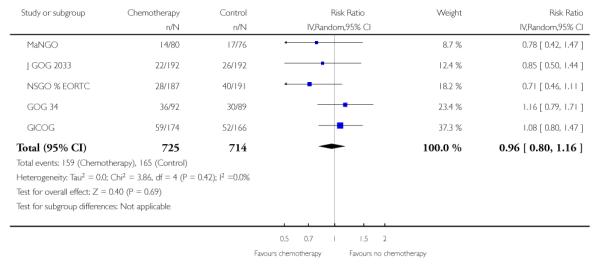 |
Analysis 3.1. Comparison 3 Separating the trials; Chemotherapy versus radiotherapy, Outcome 1 Death from any cause; Chemotherapy versus radiotherapy (HR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 3 Separating the trials; Chemotherapy versus radiotherapy
Outcome: 1 Death from any cause; Chemotherapy versus radiotherapy (HR)
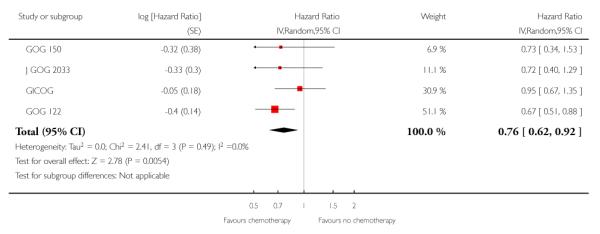 |
Analysis 3.2. Comparison 3 Separating the trials; Chemotherapy versus radiotherapy, Outcome 2 Death or recurrence; Chemotherapy versus radiotherapy (HR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 3 Separating the trials; Chemotherapy versus radiotherapy
Outcome: 2 Death or recurrence; Chemotherapy versus radiotherapy (HR)
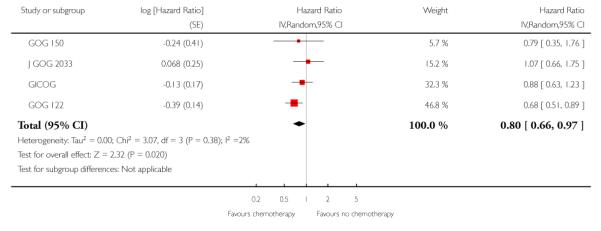 |
Analysis 3.3. Comparison 3 Separating the trials; Chemotherapy versus radiotherapy, Outcome 3 Overall 5 year survival; Chemotherapy versus radiotherapy (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 3 Separating the trials; Chemotherapy versus radiotherapy
Outcome: 3 Overall 5 year survival; Chemotherapy versus radiotherapy (RR)
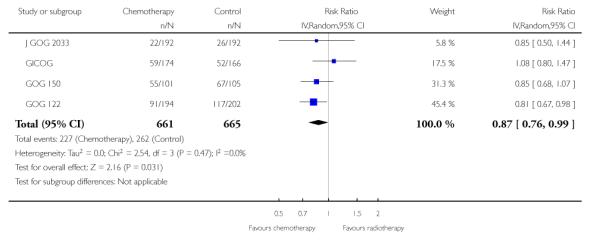 |
Analysis 4.1. Comparison 4 Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy, Outcome 1 Death from any cause; Chemotherapy versus no additional treatment (HR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 4 Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy
Outcome: 1 Death from any cause; Chemotherapy versus no additional treatment (HR)
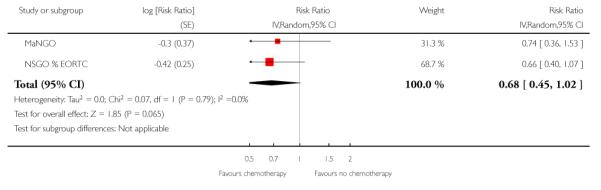 |
Analysis 4.2. Comparison 4 Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy, Outcome 2 Death or recurrence; Chemotherapy versus no additional treatment (HR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 4 Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy
Outcome: 2 Death or recurrence; Chemotherapy versus no additional treatment (HR)
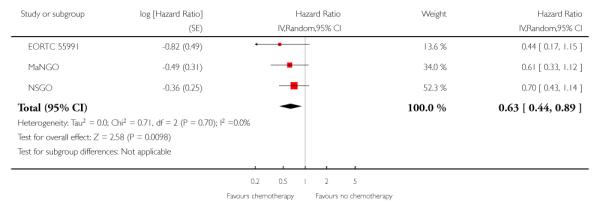 |
Analysis 4.3. Comparison 4 Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy, Outcome 3 Overall 5 year survival; Chemotherapy versus no additional treatment (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 4 Separating the trials; Chemotherapy versus no additional treatment; all women receiving surgery and radiotherapy
Outcome: 3 Overall 5 year survival; Chemotherapy versus no additional treatment (RR)
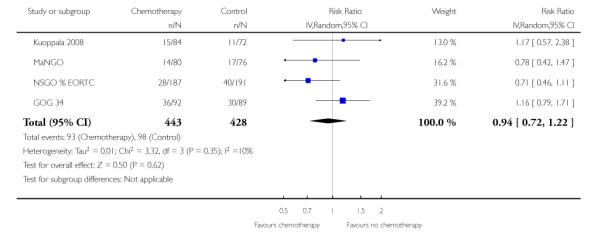 |
Analysis 5.1. Comparison 5 Sensitivity analysis, Outcome 1 Death from any cause (HR) excluding mixed tumours (carcinosarcomas)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 5 Sensitivity analysis
Outcome: 1 Death from any cause (HR) excluding mixed tumours (carcinosarcomas)
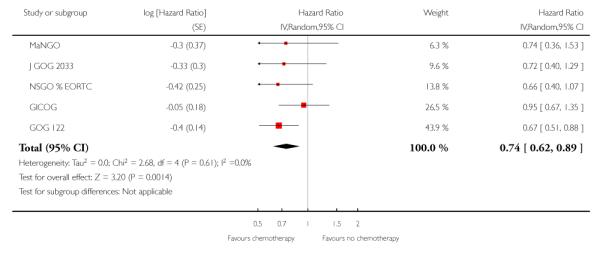 |
Analysis 5.2. Comparison 5 Sensitivity analysis, Outcome 2 Overall 5 year survival (risk of death) excluding mixed tumours (carcinosarcomas) (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 5 Sensitivity analysis
Outcome: 2 Overall 5 year survival (risk of death) excluding mixed tumours (carcinosarcomas) (RR)
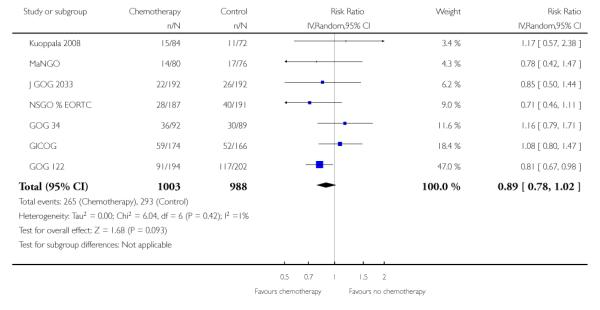 |
Analysis 5.3. Comparison 5 Sensitivity analysis, Outcome 3 Overall 5 year survival (risk of death) excluding mixed tumours and trials of unconventional chemotherapy (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 5 Sensitivity analysis
Outcome: 3 Overall 5 year survival (risk of death) excluding mixed tumours and trials of unconventional chemotherapy (RR)
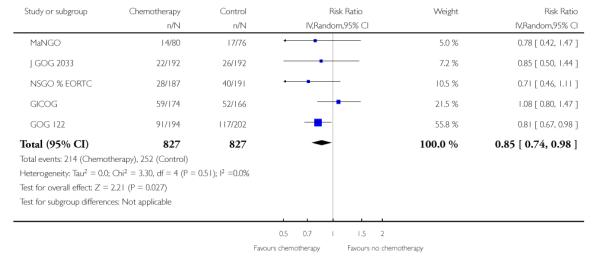 |
Analysis 6.1. Comparison 6 Site of first recurrence, Outcome 1 Distant (extrapelvic) recurrence (RR; all trials)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 1 Distant (extrapelvic) recurrence (RR; all trials)
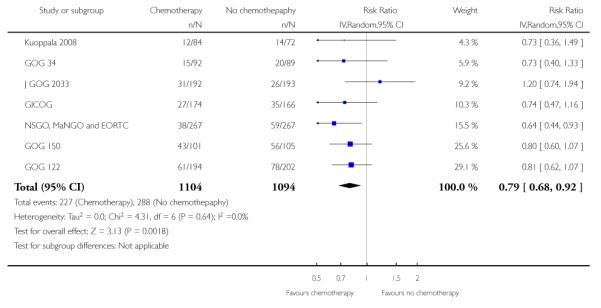 |
Analysis 6.2. Comparison 6 Site of first recurrence, Outcome 2 Distant (extrapelvic) recurrence; (Risk difference; all trials)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 2 Distant (extrapelvic) recurrence; (Risk difference; all trials)
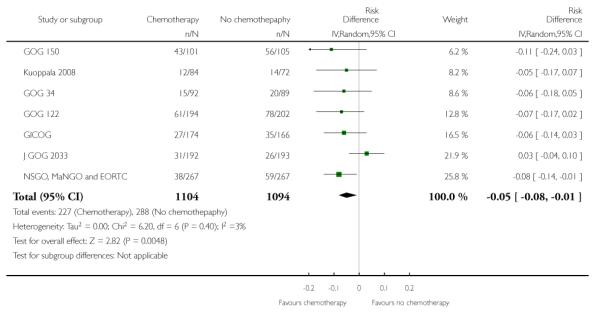 |
Analysis 6.3. Comparison 6 Site of first recurrence, Outcome 3 Distant (extrapelvic) recurrence excluding mixed tumours (RR; all trials)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 3 Distant (extrapelvic) recurrence excluding mixed tumours (RR; all trials)
 |
Analysis 6.4. Comparison 6 Site of first recurrence, Outcome 4 Pelvic recurrence (chemotherapy v no treatment) (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 4 Pelvic recurrence (chemotherapy v no treatment) (RR)
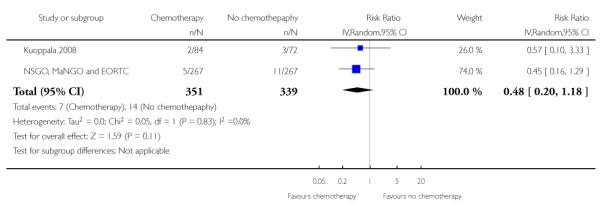 |
Analysis 6.5. Comparison 6 Site of first recurrence, Outcome 5 Pelvic recurrence (chemotherapy v radiotherapy) (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 5 Pelvic recurrence (chemotherapy v radiotherapy) (RR)
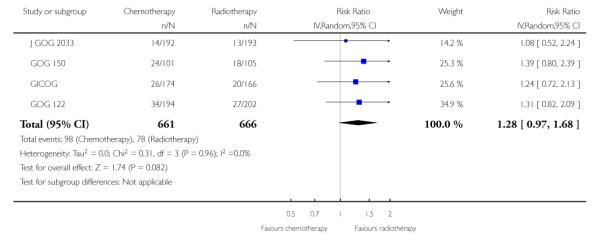 |
Analysis 6.6. Comparison 6 Site of first recurrence, Outcome 6 Any recurrence (chemotherapy v no adjuvant treatment) (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 6 Any recurrence (chemotherapy v no adjuvant treatment) (RR)
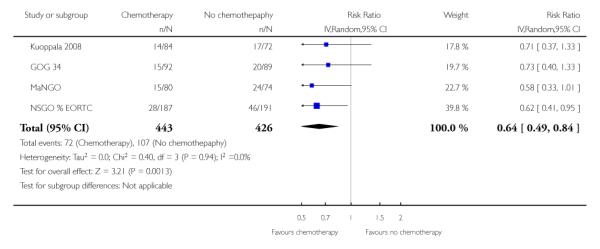 |
Analysis 6.7. Comparison 6 Site of first recurrence, Outcome 7 Any recurrence (chemotherapy v radiotherapy) (RR)
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 6 Site of first recurrence
Outcome: 7 Any recurrence (chemotherapy v radiotherapy) (RR)
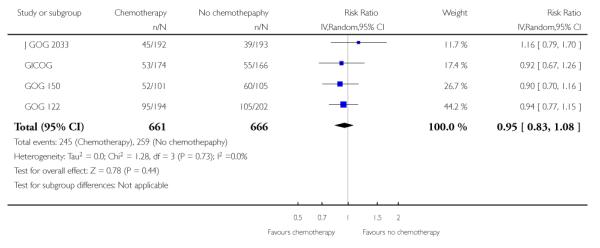 |
Analysis 7.1. Comparison 7 Subgroup analysis of NON-endometriod cancer (clear and serous), Outcome 1 Death from any cause (HR) for subgroup serous/clear cell
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 7 Subgroup analysis of NON-endometriod cancer (clear and serous)
Outcome: 1 Death from any cause (HR) for subgroup serous/clear cell
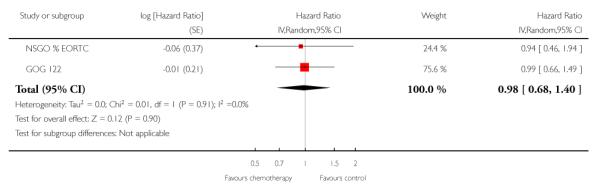 |
Analysis 7.2. Comparison 7 Subgroup analysis of NON-endometriod cancer (clear and serous), Outcome 2 Death or recurrence (HR) for subgroup serous/clear cell cancer
Review: Adjuvant chemotherapy for endometrial cancer after hysterectomy
Comparison: 7 Subgroup analysis of NON-endometriod cancer (clear and serous)
Outcome: 2 Death or recurrence (HR) for subgroup serous/clear cell cancer
 |
Appendix 1. MEDLINE search strategy
1950 to August Week 1, 2010
exp Endometrial Neoplasms/
(endometr* adj5 neoplas*).mp.
(endometr* adj5 carcinom*).mp.
(endometr* adj5 malignan*).mp.
(endometr* adj5 cancer*).mp.
(endometr* adj5 tumor*).mp.
(endometr* adj5 tumour*).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp Chemotherapy, Adjuvant/
(adjuvant adj5 chemotherap*).mp.
9 or 10
8 and 11
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
12 and 21
(animals not (humans and animals)).sh.
22 not 23
Key
mp=title, original title, abstract, name of substance word, subject heading word, unique identifierpt=publication typeab=abstractfs= floating subheadingsh=subject heading
Appendix 2. Embase Ovid search strategy
1980 to Week 32, 2010
exp Endometrium Tumor/
(endometr* adj5 neoplas*).mp.
(endometr* adj5 carcinom*).mp.
(endometr* adj5 malignan*).mp.
(endometr* adj5 cancer*).mp.
(endometr* adj5 tumor*).mp.
(endometr* adj5 tumour*).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp Adjuvant Chemotherapy/
(adjuvant adj5 chemotherap*).mp.
9 or 10
8 and 11
Controlled Clinical Trial/
Randomized Controlled Trial/
randomized.ab.
placebo.ab.
dt.fs.
randomly.ab.
trial.ab.
groups.ab.
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
12 and 21
Key
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer ab=abstract
fs=floating subheading
Appendix 3. CENTRAL search strategy
Issue 3, 2010
MeSH descriptor Endometrial Neoplasms explode all trees
endometr* near/5 neoplas*
endometr* near/5 carcinom*
endometr* near/5 malignan*
endometr* near/5 cancer*
endometr* near/5 tumor*
endometr* near/5 tumour*
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
MeSH descriptor Chemotherapy, Adjuvant explode all trees
adjuvant near/5 chemotherap*
(#9 OR #10)
(#8 AND #11)
HISTORY
Protocol first published: Issue 3, 2001
Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 6 February 2012 | Amended | Additional tables amended. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The initial protocol focused on the cure rates from endometrial cancer after hysterectomy. It was initially assumed that the participants would have cancer confined to the uterus (FIGO stage 1 or 2) but this failed to consider the fact that the FIGO stage is only diagnosed after surgery. Therefore the restriction on stage was dropped. The trial protocol also included progression-free survival as a primary outcome. This was demoted to a secondary outcome before any analysis simply because the lead author felt statistical purity demanded that there should be only one important primary outcome variable.
Before any data were collected, the lead author (NJ) decided to include the site of recurrence as a secondary outcome. NJ felt this was very important because of the related Cochrane Review of radiotherapy versus no other treatment in endometrial cancer (Kong 2007). Its addition actually has minimal impact on this review but it is important to appreciate that these data were added after the protocol had been finalised and consequently the results will have less impact.
The initial protocol restricted the inclusion criteria to endometrial cancer. Since the development of the protocol, evidence has accumulated to show that a mixed mullerian mesenchymal malignant tumour (also called carcinosarcoma) is a poorly differentiated carcinoma. If this view is accepted, mixed mullerian mesenchymal malignant tumours should be included. Consequently, the search was expanded to include these tumours in 2010. However, the analysis is represented with and without these data for readers who are not persuaded mixed mullerian mesenchymal malignant tumours should be included.
WHAT’S NEW
Last assessed as up-to-date: 31 August 2011.
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
Footnotes
DECLARATIONS OF INTEREST Nck Johnson, Andrew Bryant and Tracie Miles have no conflicts of interest.
Thomas Hogberg has participated in advisory boards for Bristol Myers Squibb and Bayer Schering Pharma
NOTES Not applicable.
References to studies included in this review
* Indicates the major publication for the study
- EORTC 55991 {unpublished data only} .Kristensen G. A randomized trial of adjuvant treatment with radiation plus chemotherapy versus radiation alone in high risk endometrial carcinoma [EORTC 55991] http://groups.eortc.be/gcg/studyprotocols.htm#55991. http://groups.eortc.be/gcg/studyprotocols.htm#55991.
- GICOG {published data only} .Maggi R, Cagnazzo G, Atlante G, Marinaccio M. Risk groups and adjuvant therapy in surgical staged endometrial cancer patients. A randomized multicentre study comparing chemotherapy with radiation therapy. International Journal of Gynecological Cancer. 1999;Vol. 99(9 Suppl 1):85. Abstract B73. [Google Scholar]; Maggi R, Cucchi L, Restelli C, Delzotti F, Frigoli A. Post surgical treatment of endometrial cancer [GICOG trial] International Journal of Gynecological Cancer. 2005;93(3 Suppl 1):46. Abstract 166. [Google Scholar]; *; Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. British Journal of Cancer. 2006;95(3):266–71. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOG 122 {published and unpublished data} .Bruner DW, Barsevick A, Tian C, Randall M, Mannel R, Cohn DE, et al. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Quality of Life Research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2007;16(1):89–100. doi: 10.1007/s11136-006-9003-5. [DOI] [PubMed] [Google Scholar]; Randall ME. ASCO media player [Whole abdominal radiotherapy versus combination doxorubicin–cisplatin chemotherapy in advanced endometrial carcinoma: A randomized phase III trial of the Gynecologic Oncology Group] 2003 http://media.asco.org/asco/meetings.education/vm/2003/slides.only/frame.asp?ID=1896&Title=Wholeabdominalradiotherapyversuscombinationdoxorubicin-cisplatinchemotherapyinadvancedendometrialcarcinoma:ArandomizedphaseIIItrialoftheGynecologicOncologyGroup&Speaker=MarcusERandall,MD&PlayThumb=0&SpeakerName=Presenter%3A%20Marcus%20E%20Randall%2C%20MD&LectureName=Whole%20abdominal%20radiotherapy%20versus%20combination%20doxorubicin-cisplatin%20chemotherapy%20in%20advanced%20endometrial%20carcinoma%3A%Key=vm.23.5.353.1896&SessionName=Plenary%20Session&mediaURL=/media&ServerName=media.asco.org.; Randall ME, Brunetto G, Muss H, Mannel RS, Spirtos N, Jeffrey F, Thigpen JT, Benda J. Whole abdominal radiotherapy versus combination doxorubicin-cisplatin chemotherapy in advanced endometrial carcinoma: A randomized phase III trial of the Gynecologic Oncology Group. Proceedings of the American Society of Clinical Oncology. 2003;Vol. 22:2. Abstract 3. [Google Scholar]; Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized Phase III Trial of Whole-Abdominal Irradiation Versus Doxorubicin and Cisplatin Chemotherapy in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2006;24(1):36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]; Thomas G. ASCO media player [Plenary Session (Dr Thomas critiquing GOG 122)] ASCO; 2003. http://media.asco.org/asco/meetings.education/vm/2003/slides.only/frame.asp?ID=3585&Title=PlenarySession&Speaker=GillianThomas,MD&PlayThumb=0&SpeakerName=Discussant%3A%20Gillian%20Thomas%2C%20MD&LectureName=A%20Randomized%20Trial%20of%20WAI%20vs.%20A%2FP%20in%20Advanced%20Endometrial%20CA&Key=vm.23.5.353.3585&SessionName=Plenary%20Session&mediaURL=/media&ServerName=media.asco.org. Vol. http://media.asco.org/asco/meetings.education/module/video/frame.asp?eventname=vm2003&ID=3585&dir=thomas&slide=01&max=15&PlayThumb=0&SpeakerName=Discussant%3A%20Gillian%20. [Google Scholar]; *; Watkins Bruner D, Barsevick A, Tian C, Randall M, Mannel R, Cohn D, et al. Quality of life trade-off to incremental gain in survival on Gynecologic Oncology Group (GOG) Protocol 122: Whole abdominal irradiation (WAI) vs. doxorubicin-platinum (AP) chemotherapy in advanced endometrial cancer. Proceedings of the American Society of Clinical Oncology. 2003;Vol. 22:449. Abstract 1803. [Google Scholar]
- GOG 150 {published data only} .GOG Protocol FAQ. Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology. 2007;107(2):177–85. doi: 10.1016/j.ygyno.2007.07.070. http://www.gog.org/protocolfaq.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOG 34 {published data only} .Morrow CP, Bundy B, Creasman W, Homesley H. A randomized study of adriamycin adjuvant chemotherapy for patients with high-risk stage I and II (occult) endometrial carcinoma: a GOG study. Proceedings, International Gynecological Cancer Society: Amsterdam, the Netherlands. 1987:87. [Google Scholar]; *; Morrow CP, Bundy BN, Homesley HD, Creasman WT, Hornback NB, Kurman R, et al. Doxorubicin as an adjuvant following surgery and radiation therapy in patients with high-risk endometrial carcinoma, stage I and occult stage II: A Gynecologic Oncology Group Study. Gynecologic Oncology. 1990;36(2):166–71. doi: 10.1016/0090-8258(90)90166-i. [DOI] [PubMed] [Google Scholar]
- J GOG 2033 {published data only} .Sagae S, Udagawa Y, Susumu N, Niwa K, Kudo R, Nozawa S. JGOG2033: Randomized phase III trial of whole pelvic radiotherapy vs cisplatin-based chemotherapy in patients with intermediate risk endometrial carcinoma. Proceedings of the American Society of Clinical Oncology.2005: 41st Annual Meeting of the American Society of Clinical Oncology; Orlando, Florida. May 13-17, 2005; 2005. Vol. Abstract 5002, issue http://media.asco.org/player/default.aspx?LectureID=1646&conferenceFolder=VM2005&SessionFolder=O24&slideonly=yes&TrackID=N929&LectureTitle=JGOG2033%3a%20Randomized%20phase%20III%20trial%20of%20Whole%20pelvic%based%20chemotherapy%20in%20patients%20with%20intermediate%20risk%20endomeKey=vm.34.5.701.1646&SpeakerName=Presenter%3a%20Satoru%20Sagae%2c%20MD%2c%20PhD&mediaURL=%2fmedia&ServerName=media.asco.org&max=24&ext=jpg&useASX=false&playtype=&playtype=&playtype=,: http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?&vmview=vm.session.presentations.view&confID=34&trackID=5&sessionID=701. [Google Scholar]; Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecologic Oncology. 2008;108(1):226–33. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Kuoppala 2008 {published data only} .Kuoppala T, Maenpaa J, Tomas E, Puistola U, Salmi T, Grenman S, et al. Surgically staged high-risk endometrial cancer: randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecol Oncol. 2008;110(2):190–5. doi: 10.1016/j.ygyno.2008.03.020. [DOI] [PubMed] [Google Scholar]
- MaNGO {unpublished data only} .*; Hogberg T, Signorelli M, Freire de Oliveira C, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer - Results from two randomised studies. European Journal of Cancer. 2010;46(13):2422–31. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSGO {published and unpublished data} .Hogberg T. Case #4 Endometrial CarcinomaStage IIIC. 2008 http://www.primeoncology.org/Files/Doc/SLIDES/EPGM06282008/Case4.pdf [Google Scholar]; Hogberg T, Rosenberg P, Kristensen G, de Oliveira CF, de Pont Christensen R, Sorbe B, et al. A randomized phase-III study on adjuvant treatment with radiation (RT) ± chemotherapy (CT) in early-stage high-risk endometrial cancer. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Abstracts of the 43rd Annual Meeting of the American Society of Clinical Oncology; Chicago, Illinois. 1-5 June 2007; 2007. p. 25. 2007. Abstract 5503. [Google Scholar]; Hogberg T, Signorelli M, Freire de, Oliveira C, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer - Results from two randomised studies. European Journal of Cancer. 2010;46(13):2422–31. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; *; Hogberg T. Presentation: A randomized phase–III study on adjuvant treatment with radiation (RT) 177; chemotherapy (CT) in early–stage high–risk endometrial cancer (NSGO–EC–9501/EORTC 55991) ASCO media player, Oral Presentation Gynecologic Cancer Track. 2007 Vol. Abstract No: 5503: http://media.asco.org/player/default.aspx?LectureID=3418&conferenceFolder=vm2007&SessionFolder=10012&slideonly=yes&TrackID=N929&LectureTitle=A%20randomized%20phase–III%20study%20on%20adjuvant%20treatment%20with%20radiation%20(RT)%20%20%20177%3b%20chemotherapy%20(CT)%20in%20early-stage%20high-risk%20endometrial%20cancer%20(NSGOEC-9501%2fEORTC%2055991).&Key=vm.47.5.364.3418&SpeakerName=Presenter%3a%20Thomas%20Hogberg%2c%20MD%2c%20PhD&mediaURL=%2fmedia&ServerName=media.asco.org&max=31&ext=jpg&useASX=false&playtype=&playtype=&playtype=,
- NSGO, MaNGO and EORTC {published data only} .*; Hogberg T, Signorelli M, Freire de Oliveira C, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer - Results from two randomised studies. European Journal of Cancer. 2010;Vol. 46(issue 13):2422–31. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSGO & EORTC {unpublished data only} .Kristensen G. Endometrial cancer [33rd ESMO Congress] 2006 http://www.esmo.org/fileadmin/media/presentations/977/1739/Endometrial%20cancer%20GBK.ppt.pdf.Stockholm
References to studies excluded from this review
- Aapro 2003 {published data only} .Aapro MS, van Wijk FH, Bolis G, Chevallier B, van der Burg MEL, Poveda A, et al. on behalf of the EORTCGCCG Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC-GCCG. Annals of Oncology. 2003;14:441–8. doi: 10.1093/annonc/mdg112. [DOI] [PubMed] [Google Scholar]
- Bruner 2007 {published data only} .Bruner DW, Barsevick A, Tian C, Randall M, Mannel R, Cohn DE, et al. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: A gynecologic oncology group study. Quality of Life Research. 2007;16(1):89–100. doi: 10.1007/s11136-006-9003-5. [DOI] [PubMed] [Google Scholar]
- Brunetto 2000 {published data only} .Brunetto V, Kilgore L, Soper J, McGehee R, Olt G, Lentz SS, et al. A phase III trial of ifosfamide alone or in combination with cisplatin in the treatment of advanced, persistent or recurrent carcinosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology. 2000;98;68(1):137. doi: 10.1006/gyno.2000.6001. Abstract 266. [DOI] [PubMed] [Google Scholar]
- Chauvergne 2008 {published data only} .Chauvergne J, Cappelaere P, Durand M, Brunet R, Hoerni B. Chemotherapy of cervico-uterin carcinoma by two associations:cisplatine or detorubicine. Results of a comparative controlled trial. Bulletin du cancer. 2008;81;68(2):363. [Google Scholar]
- Cohen 1984 {published data only} .Cohen CJ, Bruckner HW, Deppe G, Blessing JA, Homesley H, Lee JH, et al. Multidrug treatment of advanced and recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Obstetrics and Gynecology. 1984;63(5):719–26. [PubMed] [Google Scholar]
- Deng 2000 {published data only} .Deng XH, Duan W, Li BZ. Estramustine phosphate in the treatment of endometrium carcinoma. Chinese Journal of Cancer Research. 2000;12(1):58–63. [Google Scholar]
- Edmonson 1987 {published data only} .Edmonson JH, Krook JE, Hilton JF, Malkasian GD, Everson LK, Jefferies JA, et al. Randomized phase II studies of cisplatin and a combination of cyclophosphamidedoxorubicin-cisplatin (CAP) in patients with progestin-refractory advanced endometrial carcinoma. Gynecologic Oncology. 1887;28(1):20–4. doi: 10.1016/s0090-8258(87)80004-5. [DOI] [PubMed] [Google Scholar]
- Fleming 2004a {published data only} .Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of Doxorubicin Plus Cisplatin With or Without Paclitaxel Plus Filgrastim in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2004;22(11):2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- Fleming 2004b {published data only} .Fleming GF, Filiaci VL, Bentley RC, Herzog T, Vaccarello L, Gallion H. Phase III randomised trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in ednometrial carcinoma: a Gynecologic Oncology Group study. Annals of Oncology. 2004;15:1173–8. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- Fujimura 2000 {published data only} .Fujimura H, Kikkawa F, Oguchi H, Nakashima N, Mizutani S. Adjuvant chemotherapy including cisplatin in endometrial carcinoma. Gynecologic & Obstetric Investigation. 2000;50(2):127–32. doi: 10.1159/000010297. [DOI] [PubMed] [Google Scholar]
- Gallion 2003 {published data only} .Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrence endometrial carcinaoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2003;21(20):3808–13. doi: 10.1200/JCO.2003.10.083. [DOI] [PubMed] [Google Scholar]
- GCSF {published data only} .Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2004;22(11):2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- GOG 107 {published data only} .Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. Journal of Clinical Oncology. 2004;22(19):3902–8. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- GOG 156 {unpublished data only} .GOG Protocol FAQ http://www.gog.org/protocolfaq.html.
- GOG 161 {published data only} .Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2007;25(5):526–31. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- GOG 194 {unpublished data only} .GOG Protocol FAQ. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T, et al. Preliminary analysis of RTOG 9708: Adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high-risk endometrial cancer. International Journal of Radiation Oncology, Biology, Physics. 2004;59(1):168–73. doi: 10.1016/j.ijrobp.2003.10.019. http://www.gog.org/protocolfaq.html [DOI] [PubMed] [Google Scholar]
- Horton 1978 {published data only} .Horton J, Begg CB, Arseneault J, Bruckner H, Creech R, Hahn RG. Comparison of adriamycin with cyclophosphamide in patients with advanced endometrial cancer. Cancer Treatment Report. 1978;62(1):159–61. [PubMed] [Google Scholar]
- Long 1995 {published data only} .Long HJ, Nelimark RA, Cha SS. Comparison of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) vs doxorubicin and cisplatin (AC) in advanced endometrial carcinoma (Meeting abstract) Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1995;Vol. 14:282. Abstract 797. [Google Scholar]
- Omura {published data only} .Omura GA, Blessing JA, Major F, Silverberg S. A randomized trial of adriamycin versus no adjuvant chemotherapy in stage I and II uterine sarcomas. Proceedings of the American Society of Clinical Oncology. 1985;Vol. 83(2):149. doi: 10.1200/JCO.1985.3.9.1240. Abstract C-580. [DOI] [PubMed] [Google Scholar]
- Pawinski 1999 {published data only} .Pawinski A, Tumolo S, Hoesel G, Cervantes A, van Oosterom AT, Hoctin Boes G, et al. Cyclophosphamide or ifosfamide in patients with advanced and/or recurrent endometrial carcinoma: a randomised phaseII study of the EORTC Gynecological Cancer Cooperative Group. European Journal of Obstetrics and Gynecology and Reproductive Biology. 1999;86:179–83. doi: 10.1016/s0301-2115(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Samuels 2004 {published data only} .Samuels BL, Rushing D, Chawla SP, Schuetze SM, Von Mehren M, Keohan ML, et al. Randomized phase II study of trabectedin (ET-743) given by two different dosing schedules in patients with leiomyosarcomas or liposarcomas refractory to conventional doxorubicin and ifosfamide chemotherapy. Proceedings of the American Society of Clinical Oncology. 2004;Vol. 23:814. Abstract 9000. [Google Scholar]
- Sutton 2000 {published data only} .Sutton G, Brunetto VL, Kilgore L, Soper JT, McGehee R, Olt G, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology. 2000;79(2):147–53. doi: 10.1006/gyno.2000.6001. [DOI] [PubMed] [Google Scholar]
- Thigpen 1994 {published data only} .Thigpen JT, Blessing JA, DiSaia PJ, Yordan E, Carson LF, Evers C. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Journal of Clinical Oncology. 1994;12(7):1408–14. doi: 10.1200/JCO.1994.12.7.1408. [DOI] [PubMed] [Google Scholar]
- Thigpen 2004 {published data only} .Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A Gynecologic Oncology Group Study (GOG) Journal of Clinical Oncology. 2004;22(19):3902–8. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- GOG 249 {unpublished data only} .Pelvic Radiation Therapy or Vaginal Implant Radiation Therapy. Paclitaxel. Carboplatin in Treating Patients With High-Risk Stage I or Stage II Endometrial Cancer http://clinicaltrials.gov/ct2/show/NCT00807768. http://www.cancer.gov/clinicaltrials/ft-GOG-0249.
- GOG 258 {unpublished data only} .GOG 0258 (RTOG 1073 Endorsed) A Randomized Phase III Trial of Cisplatin and Tumor Volume Directed Irradiation Followed by Carboplatin and Paclitaxel vs. Carboplatin and Paclitaxel for Optimally Debulked, Advanced Endometrial Carcinoma. http://www.rtog.org/LinkClick.aspx?fileticket=7aqw-i1bTZ8%3D&tabid=331.
- Hogberg {unpublished data only} .Thomas Hogberg, MMC-08-03-060. NCT01041027 Is the sequential combination of RT after four courses of CT better then 2 additional courses of CT in the adjuvant therapy of high-risk endometrial cancer. A Phase III intergroup trial on adjuvant therapy in radically operated endometrial cancer patients with high risk for micro-metastatic disease: 4 courses of adjuvant CT (CT) followed by radiation therapy (RT) versus 2 more courses of CT.
- PORTEC 3 {unpublished data only} .CKTO-2006-04ISRCTN14387080. CKTO-PORTEC-3. EU-20664. NCT00411138 Phase III Randomized Study of Concurrent Chemoradiotherapy Followed By Adjuvant Chemotherapy Versus Pelvic Radiotherapy Alone in Patients With High-Risk Stage IB-III Endometrial Carcinoma Alternate Title. Chemotherapy and Radiation Therapy Compared With Radiation Therapy Alone in Treating Patients With High-Risk Stage I, Stage II, or Stage III Endometrial Cancer.
Additional references
- Abu-Rustum 2010 .Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Soslow RA, Levine DA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecologic oncology. 2010;116(3):399–403. doi: 10.1016/j.ygyno.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amant 2007 .Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Treatment modalities in endometrial cancer. Current Opinion in Oncology. 2007;19(5):479–85. doi: 10.1097/CCO.0b013e32827853c0. [DOI] [PubMed] [Google Scholar]
- Aoki 2004 .Aoki Y, Watanabe M, Amikura T. Adjuvant chemotherapy as treatment of high-risk stage I and II endometrial cancer. Gynecologic Oncology. 2004;94:333–9. doi: 10.1016/j.ygyno.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Butler 2004 .Butler L, Bacon M, Carey M, Zee B, Tu D, Bezjak A. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. Journal of Clinical Oncology. 2004;15(22):2461–8. doi: 10.1200/JCO.2004.01.106. [DOI] [PubMed] [Google Scholar]
- Cheong 2007 .Cheong KA, Chrystal K, Harper PG. Adjuvant chemotherapy in non-small cell lung cancer. International Journal of Clinical Practice. 2007;611(1):143–6. doi: 10.1111/j.1742-1241.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- Cochrane Gynaecological Cancer Group .Williams C, Quinn G, Jess C, Bishop T, Hayes J. Cochrane Gynaecological Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs) 2010;(issue 2) http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/GYNAECA/ [Art. No.: GYNAECA]. Cochrane Database of Systematic Reviews. [Google Scholar]
- Creutzberg 2010 .Creutzberg C. Adjuvant Chemotherapy for Endometrial Cancer. International Journal of Gynecological Cancer. 2010;20(11):S60–S63. doi: 10.1111/IGC.0b013e3181f60af6. [DOI] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition BMJ Publication Group; 2001. [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Fehr 2006 .Fehr MK, Streich MS. The validity of chemotherapy in the treatment of endometrial cancer (German) Gynakologisch-Geburtshilfliche Rundschau. 2006;46(1-2):34–8. doi: 10.1159/000089975. [DOI] [PubMed] [Google Scholar]
- Ferlay 2004 .Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0 IARCPress; Lyon: 2004. [Google Scholar]
- Ferlay 2010 .Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008 [GLOBOCAN 2008] International Journal of Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Figueredo 2008 .Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database of Systematic Reviews. 2008;(Issue 3) doi: 10.1002/14651858.CD005390.pub2. [DOI: 10.1002/14651858.CD005390.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadducci 2007 .Gadducci A, Tana R, Cosio S, Genazzani AR. Adjuvant treatment in endometrial cancer. Ginecologia y Obstetricia de Mexico. 2007;3(1):1–7. [Google Scholar]
- Galaal 2010 .Galaal K, Godfrey K, Naik R, Kucukmetin A, Bryant A. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database of Systematic Reviews. 2007;(Issue 4) doi: 10.1002/14651858.CD006812.pub2. [DOI: 10.1002/ 14651858.CD006812] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber 1995 .Gelber RD, Cole BF, Goldhirsch A, Bonadonna G, Howell A, McArdle CS, et al. Adjuvant chemotherapy for premenopausal breast cancer: a meta-analysis using quality-adjusted survival. Cancer Journal from Scientific American. 1995;1(2):114–21. [PubMed] [Google Scholar]
- GOG 108 .Sutton GP, Blessing JA, Barrett RJ, Soper JT, McGehee R, Olt G, et al. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. American Journal of Obstetrics and Gynecology. 1992;166(2):556–9. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- GOG 9001 .Reisinger SA, Asbury R, Liao SY, Homesley HD. A phase I study of weekly cisplatin and whole abdominal radiation for the treatment of stage III and IV endometrial carcinoma. Gynecologic Oncology. 1996;63(3):299–303. doi: 10.1006/gyno.1996.0326. [DOI] [PubMed] [Google Scholar]
- GOG 94 .Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecologic Oncology. 2005;97(3):755–63. doi: 10.1016/j.ygyno.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Green 2005 .Green JA, Kirwan JJ, Tierney J, Vale CL, Symonds PR, Fresco LL, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database of Systematic Reviews. 2005;(Issue 3) doi: 10.1002/14651858.CD002225.pub2. [DOI: 10.1002/ 14651858.CD002225.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa 1995 .Hayakawa S, Sato S, Takano T, Wagazuma S, Yajima A. Chemotherapy of uterine endometrial cancer [Japanese] Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1995;22(9):1169–75. [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration; [updated February 2008]. 2008. [Google Scholar]
- Hirai 2002 .Hirai M, Hirono M, Oosaki T. Adjuvant chemotherapy in stage I uterine endometrial carcinoma. International Journal of Gynaecology and Obstetrics. 2002;78:37–44. doi: 10.1016/s0020-7292(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Hogberg 2008 .Hogberg T. Adjuvant Chemotherapy in Endometrial Carcinoma: Overview of Randomised Trials. Clinical Oncology. 2008;20(6):463–9. doi: 10.1016/j.clon.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Humber 2005 .Humber C, Tierney J, Symonds P, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database of Systematic Reviews. 2005;(Issue 3) doi: 10.1002/14651858.CD003915.pub2. [DOI: 10.1002/ 14651858.CD003915.pub3] [DOI] [PubMed] [Google Scholar]
- IOG (UK) .UK-improving outcomes guidance . Guidance on Commissioning Cancer Services Improving Outcomes in Gynaecological Cancers The Manual. Vol. Catalogue number 16149. NHS Executive Health Services Directorate Wellington House London SE1 8UG. July 1999. NHS Executive Health Services Directorate Wellington House, July 1999; London SE1 8UG: http://www.cancercare.on.ca/common/pages/UserFile.aspx?serverId=6&path=/File%20Database/CCO%20Files/PEBC/pebc4-8f.pdf [Google Scholar]
- Johnson 2007 .Johnson N, Cornes P. Survival and recurrent disease after postoperative radiotherapy for early endometrial cancer (systematic review and meta-analysis) BJOG. 2007;141(11):1313–20. doi: 10.1111/j.1471-0528.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- Kodama 2007a .Kodama J, Seki N, Ojima Y. Efficacy and prognostic implications of administering adjuvant chemotherapy to patients with endometrial cancer that is confined to the uterus. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2007;131(1):76–80. doi: 10.1016/j.ejogrb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Kodama 2007b .Hayakawa S, Sato S, Takano T, Wagazuma S, Yajima A. Chemotherapy of uterine endometrial cancer. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1995;95;22(9):1169–75. [PubMed] [Google Scholar]
- Kong 2007 .Kong A, Johnson N, Cornes P, Simera I, Collingwood M, Williams C, et al. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD003916.pub2. [DOI: 10.1002/ 14651858.CD003916.pub2] [DOI] [PubMed] [Google Scholar]
- Lee 2006 .Lee CM, Szabo A, Shrieve DC, Macdonald OK, Gaffney DK. Frequency and Effect of Adjuvant Radiation Therapy Among Women With Stage I Endometrial Adenocarcinoma. JAMA. 2006;295:389–97. doi: 10.1001/jama.295.4.389. [DOI] [PubMed] [Google Scholar]
- Lukka 2006 .Lukka H, Chambers A, Fyles A, Thephamongkhol K, Elit L, Fung-Kee-Fung M, et al. Adjuvant Radiotherapy in Women with Stage I Endometrial Cancer: A Clinical Practice Guideline. A Quality Initiative of the Program in Evidence-based Care (PEBC) [Cancer Care Ontario Report] 2006 Mar 9; http://www.cancercare.on.ca/common/pages/UserFile.aspx?serverId=6&path=/File%20Database/CCO%20Files/PEBC/pebc4-10f.pdf.
- Luoma 2002 .Luoma ML, Hakamies-Blomqvist L, Sjöström J, Mouridsen H, Pluzanska A, Malmström P, et al. Physical performance, toxicity, and quality of life as assessed by the physician and the patient. Acta Oncologica. 2002;41(1):44–9. doi: 10.1080/028418602317314055. [DOI] [PubMed] [Google Scholar]
- Martin-Hirsch 1999 .Martin-Hirsch PPL, Jarvis GG, Kitchener HC, Lilford R. Progestagens for endometrial cancer. Progestagens for endometrial cancer. Cochrane Database of Systematic Reviews. 1999;(Issue 4) doi: 10.1002/14651858.CD001040.pub2. [DOI: 10.1002/14651858.CD001040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- May 2010 .May K, Bryant A, Dickinson HO, Kehoe S, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database of Systematic Reviews. 2010;(Issue Issue 1) doi: 10.1002/14651858.CD007585.pub2. Art. No.: CD007585. DOI: 10.1002/14651858.CD007585.pub2. [DOI: 10.1002/ 14651858.CD007585.pub2; : ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller 2008 .Miller DS, King LP. Gynecologic oncology group trials in uterine corpus malignancies: recent progress. Journal of Gynecologic Oncology. 2008;19(4):218–22. doi: 10.3802/jgo.2008.19.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute, USA .National Cancer Institute, USA http://www.cancer.gov/
- National Comprehensive Cancer Network .National Comprehensive Cancer Network http://www.nccn.org/clinical.asp.
- Obel 2006 .Obel JC, Friberg G, Fleming GF. Chemotherapy in endometrial cancer. Clinical Advances in Hematology & Oncology. 2006;4(6):459–68. [PubMed] [Google Scholar]
- Parmar 1998 .Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Piver 2006 .Piver MS, Lele SB, Marchetti DL, Emrich LJ. Effect of adjuvant chemotherapy on time to recurrence and survival of stage I uterine sarcomas. Journal of Surgical Oncology. 2006;38(4):233–9. doi: 10.1002/jso.2930380406. [DOI] [PubMed] [Google Scholar]
- Rodriguez 2008 .Rodriguez AO. Chemotherapy for early stage endometrial cancer? Should we be using it? Current Opinion in Obstetrics & Gynecology. 2008;20(1):1–3. doi: 10.1097/GCO.0b013e3282f3d9e8. [DOI] [PubMed] [Google Scholar]
- Sobin 2009 .Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th Edition Wiley-Blackwell: 2009. [Google Scholar]
- Stanojevic 2008 .Stanojevic Z, Djordjevic B, Todorovska I, Lilic V, Zivadinovic R, Dunjic O. Risk factors and adjuvant chemotherapy in the treatment of endometrial cancer. Journal of B.U.ON. 2008;13(1):23–30. [PubMed] [Google Scholar]
- Thomas 2003 .Thomas G. ASCO media player [Plenary Session (Dr Thomas critiquing GOG 122); A Randomized Trial of WAI vs. A/P in Advanced Endometrial CA.] ASCO; 2003. http://media.asco.org/asco/meetings.education/vm/2003/slides.only/frame.asp?ID=3585&Title=PlenarySession&Speaker=GillianThomas,MD&PlayThumb=0&SpeakerName=Discussant%3A%20Gillian%20Thomas%2C%20MD&LectureName=A%20Randomized%20Trial%20of%20WAI%20vs.%20A%2FP%20in%20Advanced%20Endometrial%20CA&Key=vm.23.5.353.3585&SessionName=Plenary%20Session&mediaURL=/media&ServerName=media.asco.org Vol. http://media.asco.org/asco/meetings.education/module/video/frame.asp?eventname=vm2003&ID=3585&dir=thomas&slide=01&max=15&PlayThumb=0&SpeakerName=Discussant%3A%20Gillian%20. [Google Scholar]
- Tierney 2004 .Tierney J. Neoadjuvant Chemotherapy for Cervical Cancer Meta-analysis Collaboration (NACCCMA) Collaboration. Neoadjuvant chemotherapy for locally advanced cervix cancer. Cochrane Database of Systematic Reviews. 2004;(Issue 1) doi: 10.1002/14651858.CD001774.pub2. [DOI: 10.1002/14651858.CD001774.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nes 2005 .van Nes JG, Nortier WR, van de Velde CJ. The 4th large meta-analysis of all trials of the treatment of operable breast cancer: increased survival after a longer follow-up][Article in Dutch] Nederlands Tijdschrift voor Geneeskunde. 2005;149(26):1978–80. [PubMed] [Google Scholar]
- Winter-Roach 2009 .Winter-Roach BA, Kitchener HC, Dickinson HO. Adjuvant (post-surgical) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database of Systematic Reviews. 2009;(Issue 3) doi: 10.1002/14651858.CD004706.pub3. [DOI: 10.1002/14651858.CD004706.pub3] [DOI] [PubMed] [Google Scholar]
- World Health Organization .World Health Organization . International Statistical Classification of Diseases and Related Health Problems [World Health Organization classification of disease] 10th Revision Version WHO; 2007. [Google Scholar]