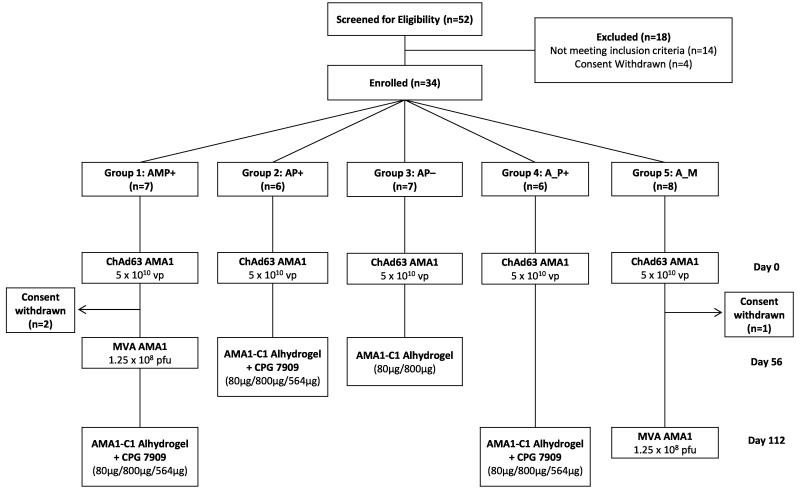
Figure 1. VAC044 flow chart of study design and volunteer recruitment.
18 volunteers were excluded following screening for the following reasons: prior malaria exposure (2 volunteers); excessive alcohol consumption (1 volunteer); proteinuria (1 volunteer); positive anti-nuclear antibody at screening (2 volunteers); nickel allergy (2 volunteers); anaemia (1 volunteer); psychiatric history (4 volunteers); haematuria (1 volunteer); consent withdrawn prior to enrolment (4 volunteers). All immunizations were administered intramuscularly with sequential vaccines administered into the deltoid of alternating arms. Two volunteers in Group 1 withdrew from the study 56 days post-ChAd63 AMA1 for personal reasons. One volunteer in Group 5 withdrew from the study 57 days post-ChAd63 AMA1 for personal reasons and was replaced with a new volunteer, thus n=8 recruited into this Group. Throughout the paper the immunization regimens are referred to as defined in the Group boxes, e.g AMP+ = ChAd63 prime, MVA boost, AMA1-C1 protein-in-Alhydrogel + CP7909 boost with 8 week intervals; A_P+ = ChAd63 prime, AMA1-C1 protein-in-Alhydrogel + CP7909 boost with a 16 week interval. Where the “AM” regimen is referred to, this relates to ChAd63 prime, MVA boost with an 8 week interval from Group 1 (before the protein vaccine boost).