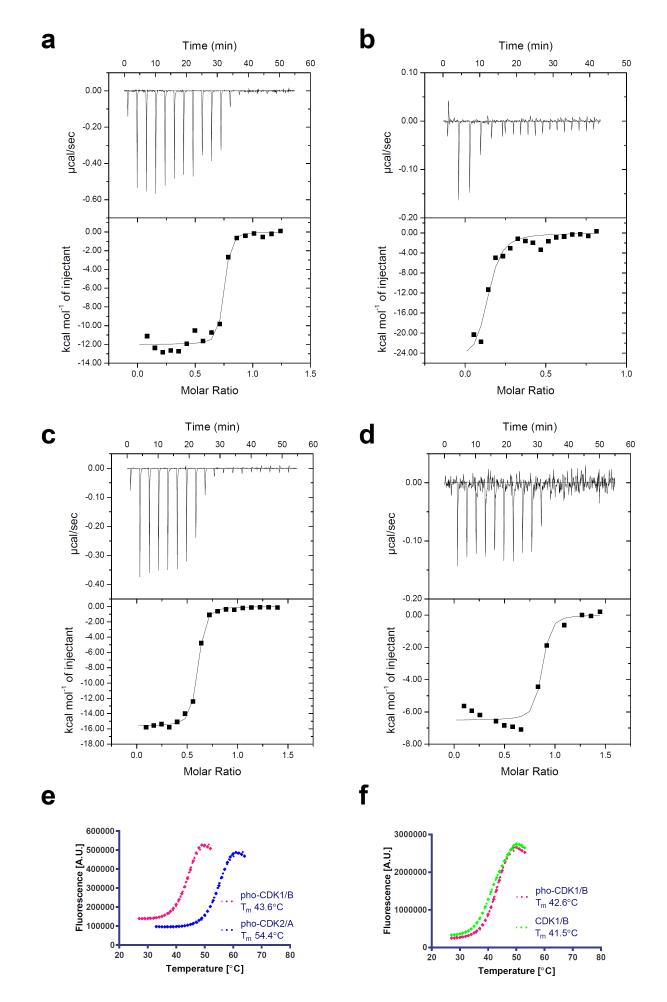
Figure 4. In vitro stability of the CDK1-cyclin B and CDK2-cyclin A complexes.
(a-d) Isothermal titration calorimetry thermograms to assess the formation of CDK2-cyclin A (a, d) and CDK1-cyclin B (b, c) complexes under conditions of lower salt, lower pH (40 mM HEPES, pH 7.4, 200 mM NaCl, 0.01% monothioglycerol) (a, b) and higher salt, higher pH (50 mM HEPES, pH 8.0, 500 mM NaCl, 0.01% monothioglycerol) (c, d). (e) Comparison of the thermal stability of the phosphorylated CDK1-cyclin B and CDK2-cyclin A complexes as assessed by differential scanning fluorimetry. (f) Comparison of the thermal stability of phosphorylated CDK1-cyclin B after either treatment with λ phosphatase, or mock-treatment under identical conditions. (e,f) Representative melting curves are shown from two replicates.