Table 1. Identification of the most active organocatalyst and best-suited reaction conditions for the asymmetric synthesis of 3a.
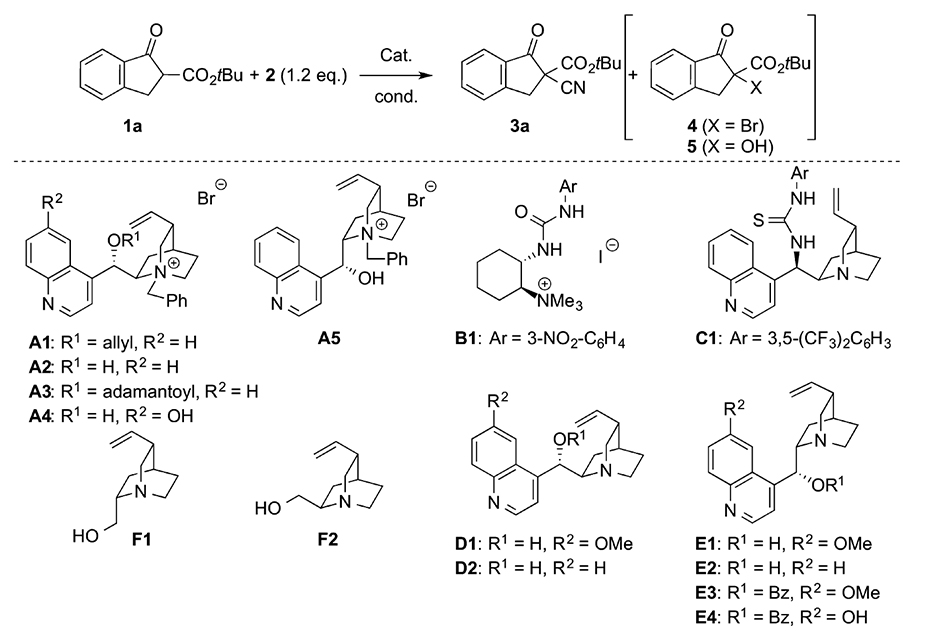
| Entry | Cat. (mol %) | Solvent | Base | T (°C) | t (h) | 3aa (%) | 4b (%) | erc (±) |
|---|---|---|---|---|---|---|---|---|
| 1 | A1 (20%) | CH2Cl2 | K2CO3 (5 equiv) | 25 | 24d | 58 | 10–15 | 48:52 |
| 2 | A1 (20%) | CH2Cl2 | – | 25 | 24 | 60 | 15–20 | 48:52 |
| 3 | A2 (20%) | CH2Cl2 | – | 25 | 24d | 30 | 15–20 | 55:45 |
| 4 | A3 (20%) | CH2Cl2 | – | 25 | 24 | 58 | 15–20 | 50:50 |
| 5 | A4 (20%) | CH2Cl2 | – | 25 | 24d | 20b | 15–20 | 50:50 |
| 6 | A5 (20%) | CH2Cl2 | – | 25 | 24d | 39 | 20 (17)a | 42:58 |
| 7 | A5 (20%) | CH2Cl2 | K2CO3 (5 equiv) | 25 | 24d | 47 | 15–20 (15)a | 44:56 |
| 8 | B1 (10%) | CH2Cl2 | – | 25 | 40d | 39 | n.d. | 37:63 |
| 9 | C1 (20%) | Toluene | – | 25 | 40d | 25 | n.d. | 34:66 |
| 10 | D1 (20%) | CH2Cl2 | – | 25 | 40 | 80 | n.d. | 64:36 |
| 11 | D2 (20%) | CH2Cl2 | – | 25 | 40 | 78 | n.d. | 66:34 |
| 12 | E1 (20%) | CH2Cl2 | – | 25 | 40 | 80 | n.d. | 38:62 |
| 13 | E2 (20%) | CH2Cl2 | – | 25 | 40 | 83 | n.d. | 30:70 |
| 14 | E3 (20%) | CH2Cl2 | – | 25 | 40 | 79 | n.d. | 50:50 |
| 15 | E4 (20%) | CH2Cl2 | – | 25 | 40 | 70 | n.d. | 42:58 |
| 16 | F1 (20%) | CH2Cl2 | – | 25 | 40d | 66 | n.d. | 50:50 |
| 17 | F2 (20%) | CH2Cl2 | – | 25 | 40d | 67 | n.d. | 47:53 |
| 18 | E2 (20%) | Toluene | – | 25 | 40d | 62 | n.d. | 33:67 |
| 19 | E2 (20%) | MTBE | – | 25 | 40 | 78 | n.d. | 41:59 |
| 20 | E2 (20%) | CHCl3 | – | 25 | 40 | 70e | n.d. | 26:74 |
| 21 | E2 (10%) | CHCl3 | – | 25 | 40d | 34 | n.d. | 26:74 |
| 22 | E2 (5%) | CHCl3 | – | 25 | 40d | 32 | n.d. | 34:66 |
| 23 | E2 (40%) | CHCl3 | – | 25 | 40 | 85 | n.d. | 30:70 |
| 24 | E2 (20%) | CHCl3 | – | 0 | 72d | 52 | n.d. | 25:75 |
| 25 | E2 (20%) | CHCl3 | – | 40 | 40 | 57 | n.d. | 27:73 |
Isolated yield.
Determined by 1H NMR of the crude reaction product.
Determined by HPLC using a chiral stationary phase.
Less than 90% conversion of 1a.
Using 2 equiv of 2 gave 3a in 75% yield and the same enantioselectivity.
