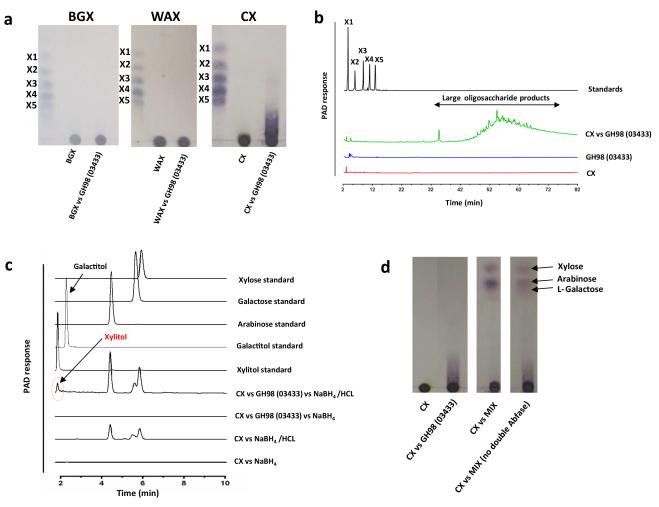
Figure 4. The activity of the GH98 enzyme BACOVA_03433.
a, Different xylans at 1% (w/v) were incubated with 1 μM of the GH98 enzyme in PBS for 16 h at 37 °C. The reaction products were subjected to thin layer chromatography and compared to xylooligosaccharide standards with a d.p. ranging from 1 to 5 (X1-X5). b. HPAEC-PAD trace showing the high molecular weight oligosaccharide products of CX digestion by the GH98 endo-xylanase, separated using a sodium acetate gradient of 0-100%. The reaction conditions used are the same as in panel a. Control reactions of substrate and enzyme only are shown. c, CX was incubated with 5 μM of the GH98 enzyme for 24 h in 50 mM sodium phosphate buffer, pH 7.0, at 37 °C. The reaction products, and CX that was not incubated with the GH98 enzyme, were then treated with sodium borohydride, to convert the reducing end monosaccharide unit into its alditol, followed by acid hydrolysis to convert the oligosaccharides (or in the case of control CX alone) into their monosaccharide constituents. The hydrolysed products were subjected to HPAEC and compared to the migration of standard monosaccharides as well as the alditol of xylose (xylitol) and galactose (galactitol). d, The GH98 enzyme was incubated with CX (lane CX vs GH98), or CX that had been pre-treated with all the side chain cleaving enzymes encoded by PUL-XylL (lane CX vs MIX), or CX that had been pre-treated with all the side chain cleaving enzymes encoded by PUL-XylL, except the GH43 enzyme BACOVA_03417 that removes O3 linked arabinose from double substituted xylose – see Supplementary Fig. 5 (lane CX vs MIX no double Abf’ase).