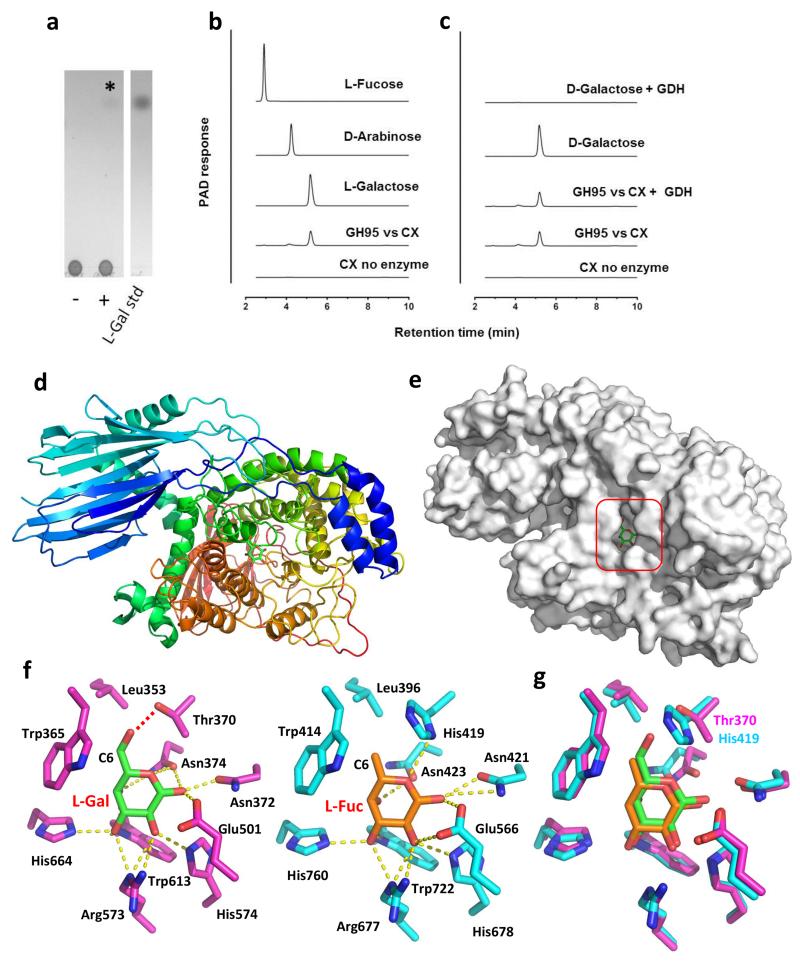
Figure 5. Activity and structure of BACOVA_03438 GH95 α-L-galactosidase and comparison to the Bifidobacterium bifidum GH95 α-L-fucosidase.
a,b TLC and HPAEC profiles, respectively, of the reaction product (L-galactose) generated by incubating CX at 1 mg/ml with 1 μM BACOVA_03438 for 16 h in 50 mM sodium phosphate buffer, pH 7.0, at 37 °C. In a the lanes are as follows: (−) CX no enzyme; (+) CX incubated with BACOVA_03438. The asterisk marks the position of the single reaction product released from CX by the GH95. c, The product of the GH95 is resistant to oxidation by d-galactose dehydrogenase (GDH). d, Schematic of BACOVA_03438, colour-ramped from blue (N-terminus) to red (C-terminus), with l-Gal bound in the active site (carbohydrate carbons shown as green sticks). e, Solvent exposed surface representation of B. ovatus GH95. The active site pocket that houses β-l-Gal product is boxed. f, Side chains of the active site residues of BACOVA_03438 (magenta carbons) and the GH95 α-l-fucosidase (blue carbons; PDB: 2EAE) from Bifidobacterium bifidum, bound to l-Gal (green carbons) and l-Fuc (orange carbons), respectively. Predicted hydrogen bonds between the amino acid side chains and carbohydrates are shown as yellow dotted lines, except for the H-bond between the Oγ of BACOVA_03438 Thr337 and O6 of l-Gal, which is shown as a red dotted line. g, Overlay of the active site residues displayed in panel f with the side-chains of the Thr/His polymorphism that may play a role in specificity for l-Gal over l-Fuc labeled. A stereo image of a portion of the electron density map is shown in Supplementary Figure 9.