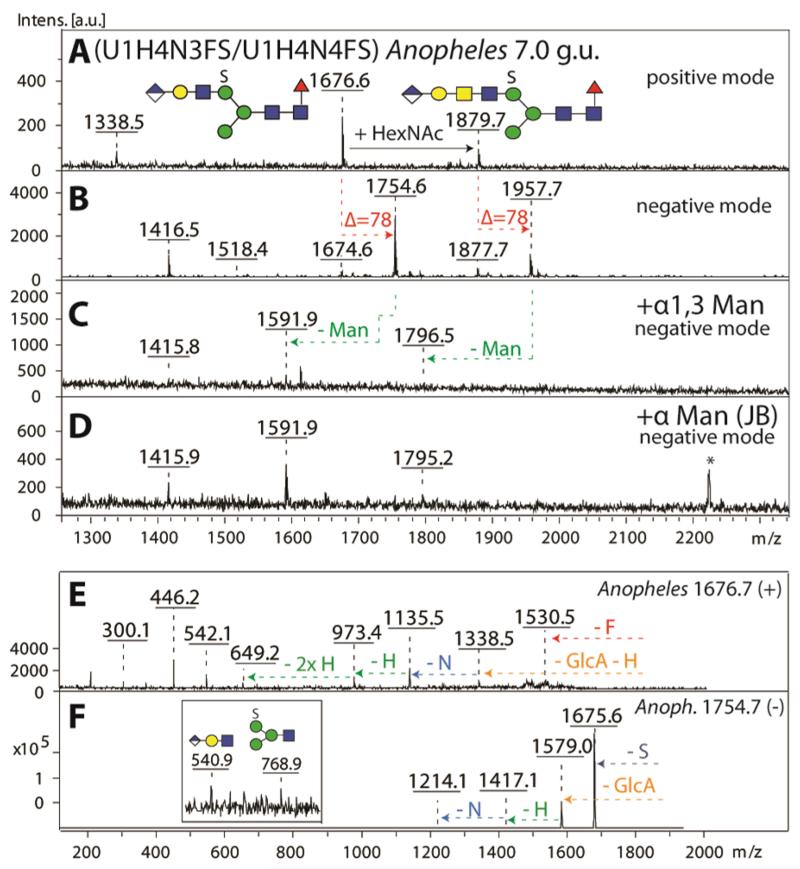
Figure 10. Definition of the antennal isomeric status of a N-glycan modified with both sulphate and glucuronic acid.
A selected RP-Amide HPLC fraction (7.0 g.u.) of PNGase F released anionic N-glycans from Anopheles was analysed in positive (A) and negative (B) ion modes before (A, B) and after exoglycosidase treatments (C, D). As judged by negative ion mode MS, both the α1,3-specific (C) and the jack bean α-mannosidases (D) were able to liberate one mannose residue from the parent glycan species (m/z 1754 and 1957). MS/MS of the most abundant parent glycan species in panel A was performed in positive (m/z 1676, E) and negative (m/z 1754, F) ion modes and offered further evidence for the structure of the backbone as well as for the position of the anionic modifications. The asterisk in (D) indicates a contaminating signal from the jack bean enzyme preparation.