Abstract
OBJECTIVES
Adolescents living with HIV face substantial difficulties in accessing HIV care services and have worse treatment outcomes than other age groups. The objective of this review was to evaluate the effectiveness of service delivery interventions to improve adolescents’ linkage from HIV diagnosis to antiretroviral therapy (ART) initiation, retention in HIV care and adherence to ART.
METHODS
We systematically searched the Medline, SCOPUS and Web of Sciences databases and conference abstracts from the International AIDS Conference and International Conference on AIDS and STIs in Africa (ICASA). Studies published in English between 1st January 2001 and 9th June 2014 were included. Two authors independently evaluated reports for eligibility, extracted data and assessed methodological quality using the Cochrane risk of bias tool and Newcastle–Ottawa Scale.
RESULTS
Eleven studies from nine countries were eligible for review. Three studies were randomised controlled trials. Interventions assessed included individual and group counselling and education; peer support; directly observed therapy; financial incentives; and interventions to improve the adolescent-friendliness of clinics. Most studies were of low to moderate methodological quality.
CONCLUSIONS
This review identified limited evidence on the effectiveness of service delivery interventions to support adolescents’ linkage from HIV diagnosis to ART initiation, retention on ART and adherence to ART. Although recommendations are qualified because of the small numbers of studies and limited methodological quality, offering individual and group education and counselling, financial incentives, increasing clinic accessibility and provision of specific adolescent-tailored services appear promising interventions and warrant further investigation.
Keywords: HIV, adolescents, antiretroviral therapy, retention, adherence linkage, systematic review
Introduction
An estimated 2.1 million adolescents (people aged between 10 and 19 years old) are infected with HIV [1], with the majority (85%) living in sub-Saharan Africa [1]. Adolescents are an underserved group in global and national responses to the HIV epidemic [2, 3], and, to date, global declarations, commitments and targets have failed to address their specific needs [1]. Adolescents may be infected with HIV through sexual contact or exposure to unsafe injections (horizontal transmission); or in utero, during delivery, or whilst breastfeeding as an infant (vertically acquired) [4]. About one-third of infants who are infected with HIV will survive into adolescence without treatment [5]. This means that large numbers of infants who were infected perinatally with HIV early in the epidemic before antiretroviral prevention of mother-to-child transmission was widely available are now surviving in adolescence due to increased availability of antiretroviral therapy (ART).
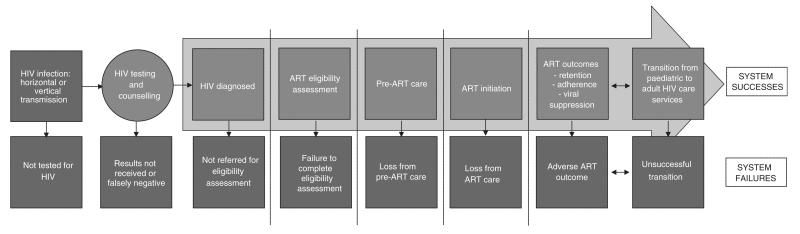
Adolescents infected with HIV face particular challenges accessing effective care and achieving successful treatment outcomes (Figure 1). Adolescents undergo a period of rapid physical, emotional and behavioural change [3]. They frequently face substantial socio-economic problems, which may be accentuated if they are orphaned or looked-after by social care services [11]. Adolescents who were infected with HIV perinatally may have health and developmental problems including opportunistic infections, stunting, chronic lung disease and neuropsychiatric complications [2]. There is little specific provision for adolescents within the health systems of most countries, with this group often falling into the gaps between paediatric and adult services. Problems related to the transition between paediatric and adult care are common [3] and include adolescents’ fear of leaving supportive paediatric care, competing demands with higher education, starting employment and moving out of the family home, and insufficient proactive transition planning by health services, all of which may contribute to periods of worsened HIV care outcomes [6].
Figure 1.
The adolescent-focused HIV care pathway. Based on a figure by Kranzer and colleagues [79] (adapted).
The few studies that have investigated HIV-infected adolescents’ progression through the HIV care pathway have generally found their outcomes to be poor and worse than for adults or young children. Rates of retention in care prior to antiretroviral therapy (ART) initiation have been reported to be substantially worse for adolescents than for adults [7]. Once ART has been initiated, retention [8] and adherence to treatment are also poor, with a recent systematic review showing that, although studies used different measurement approaches, an average of only 62% of 12- to 24-year olds achieved 95% or greater adherence [9].
Interventions to ensure adolescents’ prompt linkage into HIV care and high levels of retention and adherence to ART could have substantial individual and public health benefits [10–12], but must be responsive to their unique needs. This study reviewed the effectiveness of service delivery interventions aimed at improving adolescents’ linkage into HIV care and their subsequent retention and adherence to ART.
Methods
Study design
Systematic review.
Inclusion and exclusion criteria
This review included studies published between 1st January 2001 and 9th June 2014. We sought studies that reported on the effect of service delivery interventions on adolescents’ linkage from HIV diagnosis to ART initiation, retention on ART and adherence to ART. Both randomised controlled trials and non-randomised studies (cohort studies, case–control studies, before and after and time-series studies, and reports of routinely collected programme data) were included. Reviews, commentaries, editorials, case studies, case series, economic analyses, mathematical modelling studies and qualitative studies were excluded.
Adolescents were defined as study participants aged between 10 and 19 years. Studies were included if the majority (>50%) of study participants were adolescents. Studies that reported on mixed cohorts (young adults and adolescents, or older children and adolescents) were included if the mean or median age of the participants was between 10 and 19 years of age, or if the majority (>50%) of participants were aged between 10 and 19 years. In other studies, where the effect of interventions on participants with a wider range of ages was investigated, we included studies where age-disaggregated data relevant to adolescents were reported.
Service delivery interventions [13] were defined to be interventions provided through health systems or health providers with the objective of improving adolescents’ linkage from HIV diagnosis to ART initiation, retention on ART or adherence to ART. Studies that reported exclusively on the effect of one or more specific clinical treatment options (such as specific ART regimens or combinations) were excluded.
Completion of steps on the HIV care pathway between HIV diagnosis and initiation of ART was defined to include any of completion of assessment of ART eligibility (clinical staging, CD4 count measurement); completion of educational sessions and training; continuous collection of prophylactic therapy; and initiation of ART. In line with WHO recommendations on terminology [14], we defined retention on ART as continuous engagement in ART treatment, with retained participants being adolescents who attended at least one scheduled treatment appointment within the last 90 days. Adherence to ART was defined as regularly taking all antiretroviral medications that made up the prescribed ART regimen in the correct dose and at the correct time. Studies used a variety of outcome measures to define these outcomes of interest. Where possible, we used WHO-recommended definitions for each of the three key outcomes (linkage to ART, retention, adherence). Where other outcome measures were used, we report outcomes as defined by the study’s authors.
We placed no restrictions on countries studied. Only studies published in English were included.
Search strategy
We searched the Medline (via PubMed), SCOPUS and Web of Sciences databases following a pre-defined search strategy included within our published study protocol registered in the University of York database for Prospectively Registered Systematic Reviews in Health and Social Care (PROSPERO) [15]. Search terms and full search strategies are shown in Figure S1. We also searched the online databases of conferences abstracts from the International AIDS Conferences (2006–2014) and the International Conference on AIDS and STDs in Africa (Dates 2008–2013). The bibliographies of selected studies were reviewed by hand to identify studies that may have been missed by our search strategy.
Selection of studies
After exclusion of duplicate studies, two reviewers (PM and CM) independently screened the title and abstracts of all retrieved studies to identify studies eligible for full text review. Discrepancies were resolved by discussion between the reviewers, with arbitration by a third reviewer (DR) where agreement could not be reached.
A data extraction form, which had been pilot tested on a sample of manuscripts, was completed independently by two reviewers (PM and CM) for all studies selected for full text review. Reviewers recorded the presence of inclusion or exclusion factors, and if the study was eligible for inclusion, the following information was recorded from each study: year of study; study setting and population; description of study design and intervention(s) investigated; and outcome measures assessed. For each study with data available, the proportion (and 95% confidence interval [CI]) of adolescents completing steps on the HIV care pathway, retained on ART and adherent to ART, were recorded. Where these data were not available, study-reported outcomes were extracted. Where a control or comparison group was evaluated, the relative and absolute effect of the intervention and 95% CIs were recorded, where available. If duplicate or partially duplicated results were presented in more than one study, we extracted data from both studies and included only the study with the most complete data.
Two reviewers (PM and CM) undertook an assessment of methodological quality for each study. For randomised controlled trials, the Cochrane Collaboration’s Tool for Assessing Risk of Bias was used [16]. For non-randomised studies, a modified version of the Newcastle–Ottawa Scale was used [17]. The Newcastle–Ottawa Scale was modified by removing items relating to procedures for selection of the non-exposed cohort, as most studies did not include a control group. Given the possibility of highly heterogeneous study designs, an additional item was added to assess the presence any other potential sources of bias such as the size of the study and reported fidelity to study interventions. Results were compared and a consensus overall risk of bias judgement was made for each study. Randomised studies were graded as providing good (score 5–6), moderate (score 3–4) or low quality of evidence (0–2). Non-randomised studies were graded as providing good (score 7–10), moderate (score 4–6) or low quality of evidence (score 0–3). An overall judgement of ‘unclear risk of bias’ was made where there was inadequate information provided by the study report.
Because we anticipated that studies would come from a wide variety of settings and populations, and report on a range of different interventions, we expected that there would be a high degree of heterogeneity. We therefore did not plan to attempt to estimate summary measures of effectiveness for interventions, but would have done so if the review indicated this to be appropriate.
Results
Study characteristics
The search identified 3138 unique abstracts, from which 3070 were excluded after review of title and abstract (Figure S2). Seventy-four manuscripts were reviewed in full, of which 11 studies met the review’s inclusion criteria [18–28]. The main reason for exclusion was age (41/63, 65% of exclusions), with studies including a majority of participants aged <10 years old or >19 years old, and not reporting age-disaggregated data to allow extraction data for all or part of the 10- to 19-year age group (Table S2 – reasons for exclusion).
Characteristics of the 11 included studies are summarised in Table S1. Studies were conducted between 1998 and 2012. Six studies were from the USA [18, 20, 23, 26–28], one from each of the UK [21], France [22], South Africa [19] and Thailand [24], and one study was a multicentre study conducted in Kenya, Mozambique, Rwanda and Tanzania [25]. Three studies were randomised controlled trials [18, 19, 26], and eight were nonrandomised studies with a variety of study designs, including prospective [21, 22, 24] and retrospective cohort studies [20, 23], and reports of routinely collected programme data [25, 27, 28].
The number of participants included in studies ranged between 9 [23] and 57 038 [25]. The proportion of adolescent study participants that were male ranged between 25% [21] and 60% [20]. There was considerable variation between studies in definitions of ‘adolescence’, ‘youth’ and ‘young adult’. Three studies specifically included key populations, including men who have sex with men [20, 28] and injecting drug users [20, 26].
Study settings comprised HIV care clinics (n = 9 studies [18–22, 24–27], two of which described clinics as being specialist or dedicated adolescent centres [18, 21]), a children’s hospital [23], and an inpatient rehabilitation facility for children and adolescents affected by HIV [28]. Two distinct groups of study participants were included in the different studies: some only included HIV-infected adolescents meeting defined criteria for treatment failure or suboptimal adherence [21, 23, 24, 26–28], whilst others included HIV-infected adolescents, selected irrespective of treatment success/failure or adherence [18–20, 22, 25].
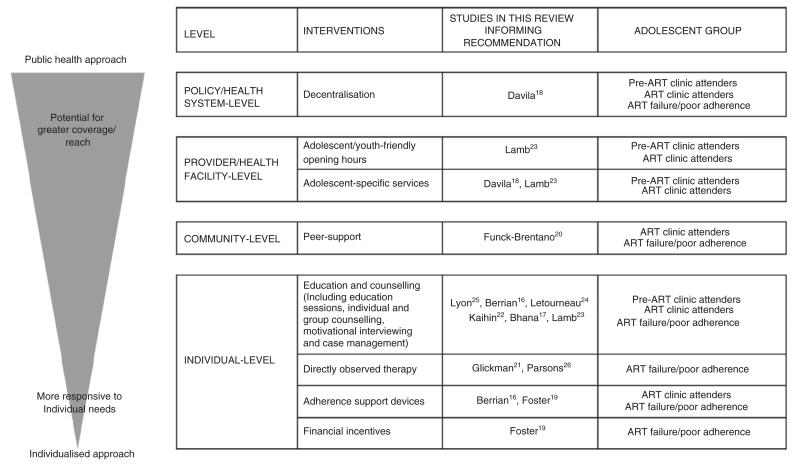
In the eleven studies included in the review, a total of eight interventions were evaluated. We classified interventions as having been applied at the individual-, community-, provider and health facility-, and policy and health system levels (Figure 2).
Figure 2.
Typography of interventions to improve adolescents’ linkage, retention and adherence to ART. Where studies evaluated interventions consisting of more than one component, or complex multifaceted interventions, they are listed more than once in the figure.
Interventions applied at the individual level (education and counselling, directly observed therapy, adherence support devices and financial incentives) and community level (peer support) tended to be intensive, applied in multiple sessions over weeks to months, and were personally tailored to be responsive to the needs of individual adolescents. In contrast, interventions applied at the provider and health facility-level (adolescent/youth friendly clinic opening hours, provision of adolescent-specific services) and policy and health system level (decentralisation) emphasised individual patient needs less, but had greater potential to reach a larger number of adolescents and used fewer resources than individual-level approaches.
Linkage to HIV care and ART
Only one study evaluated interventions to improve adolescents’ linkage to HIV care and ART [25] (Table 1a). Routinely collected clinic and patient data from a multisite study in four countries in sub-Saharan Africa were used to evaluate the effect of availability of clinic interventions on pre-ART attrition. At the clinic-level, two sets of interventions were either available or not available: adolescent-targeted services (defined as clinics that had dedicated adolescent clinics and opening hours, peer educators, or support groups) and services likely to be used by youth (clinics with screening for sexually transmitted infections, provision of condoms and hormonal contraceptives, and education). In adjusted analysis, none of the available clinic adolescent-targeted services likely to be used by youth were associated with risk of pre-ART attrition.
Table 1.
Summary of findings – interventions to (a) improve adolescents’ retention in pre-ART care and linkage to ART, (b) improve adolescents’ retention on ART and (c) improve adolescents’ adherence to ART
| Study | Outcome evaluated |
Intervention or exposure |
Number receiving intervention |
Number with missing outcome |
Number with outcome of interest (%) |
Comparison | Number receiving comparison |
Number with missing outcome |
Number with outcome of interest (%) |
Relative effect (95% CI) of intervention |
P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||||
| Lamb [25]* | Cumulative incidence of attrition from pre-ART care after 1 year | Adolescent-specific clinic opening hours | NR† | NR | NR | No specific adolescent clinic opening hours | NR† | NR | NR | 1.06 (0.89–1.27)¥ | NR |
| (b) Studies including all HIV-infected adolescents attending clinics | |||||||||||
| Davila [20] | Proportion of participants with at least 3 clinic visits during 1 year follow-up | a. Centralised and enhanced youth support activities | 48 | NR | 31 (64.5%) | b. Centralised care with youth multidisciplinary clinics | 90 | NR | 51 (56.7%) | (a vs. b): 1.18 (0.55–2.53)‡ | 0.68 |
| c. Decentralised care only | 36 | NR | 11 (30.6%) | (c vs. b): 0.42 (0.17–1.03)‡ | 0.06 | ||||||
| Proportion of participants with no gaps in care of >180 days during 1 year follow-up | a. Centralised and enhanced youth support activities | 48 | NR | 46 (95.8%) | b. Centralised care with youth multidisciplinary clinics | 90 | NR | 72 (80.0%) | (a vs. b): 5.56 (1.20–25.0)‡ | 0.03 | |
| c. Decentralised care only | 36 | NR | 30 (83.3%) | (c vs. b): 1.37 (0.46–4.17)‡ | 0.57 | ||||||
| Lamb [25] | Cumulative incidence of attrition from ART after 1 year | Adolescent-specific clinic opening hours | NR† | NR | NR | No specific adolescent clinic opening hours | NR† | NR | NR | 1.48 (1.05–2.08)¥ | NR |
| (c) Studies including HIV-infected adolescents attending clinics, who had previous problems with retention or adherence, or poor treatment outcomes | |||||||||||
| Foster [21] | 1. Change in median HIV viral load between baseline and 12 months | Financial incentives linked to HIV viral load results, motivational interviewing, provision of adherence support devices | 11 | 0 (0%) | Median at baseline: 12 900 copies/ml Median at 12 months: 105 copies/ml | No comparison group | – | – | – | – | – |
| 2. Change in median HIV viral load between baseline and 24 months | 11 | 1 (9.1%) | Median at baseline: 12 900 copies/ml Median VL at 24 months: <50 copies/ml | – | – | – | – | – | |||
| Glikman [23] | 1. Mean change in HIV viral load from pre- DOT to completion of DOT | Inpatient DOT for 7 days, supported by education from physicians, nurses, nutrition specialists and social workers | 13 admissions for 9 patients | 2 (15.4%) | Mean change (SD): −0.8 (0.55) | No comparison group | – | – | – | – | 0.04 |
| 2. Change in mean HIV viral load from pre-DOT to 6-months post-DOT | 13 admissions for 9 patients | NR | NR | No comparison group | – | – | – | – | NR (‘not significant’) | ||
| Kaihin [24] | Proportion of participants achieving >95% adherence 8 weeks after last empowerment group session | Empowerment building weekly group sessions | 23 | 0 (0%) | Baseline: 88.0% Follow-up: 97.4% | NR | 23 | 0 (0%) | Baseline: 88.0% Follow-up: 89.9% | NR | NR∑ |
| Letourneau [26] | Between-group comparison of rate of change in mean ‘medication adherence score’§ over 9 months | Multisystematic therapy provided for 6-months | 20 | NR | NR | Usual care with motivational interviewing and financial incentives for attendance | 14 | NR | NR | OR: 0.93 (no CI presented)¶ | 0.693 |
| Lyon [27] | Before and after comparison of change in responses to National Institute of Health Adherence to Medication Questionnaire | 12-week multidisciplinary family curriculum | 18 | 0 (0%) | Skipped at least one dose in the past 2 weeks: Before: 78%, After: 36% Skipped at least one dose yesterday: Before: 50%, After: 12% Skipped at least one dose in the past 2 days Before: 43%, After: 18% | No comparison group | – | – | – | – | – |
| Parsons [28] | 1. Change in mean HIV viral load (log10) between admission and discharge | DOT provided with multidisciplinary inpatient care | 19 | 0 (0%) | Admission: 5.76 Discharge: 4.77 | No comparison group | – | – | – | – | <0.001 |
| 2. Change in mean HIV viral load (log10) between admission and 6 months after discharge | DOT provided with multidisciplinary inpatient care | 19 | 0 (0%) | Admission: 5.76 6 months: 5.05 | No comparison group | – | – | – | – | 0.004 | |
| Studies including all HIV-infected adolescents attending clinics | |||||||||||
| Berrian [18] | 1. Mean difference in rate of collection of monthly prescribed ART, averaged over 3 months and ascertained from pharmacy records | 8 structured nurse home visits over 3 months, with provision of education and adherence support devices | 20 | 1 (5.0%) | NR | Clinic-based adherence advice with provision of adherence support devices | 19 | 2 (10.5%) | NR | NR | 0.002 |
| 2. Mean difference in self-reported adherence (not further defined) | 8 structured nurse home visits over 3 months, with provision of education and adherence support devices | 20 | NR | Mean difference (SE) in adherence score (0–3 months): +2.78 (0.88) | Clinic-based adherence advice with provision of adherence support devices | 19 | NR | Mean difference (SE) in adherence score (0–3 months): +0.2 (0.96) | NR | 0.07 | |
| Bhana [19] | Change in ‘missed last ART dose’** using paediatric AIDS clinical trials group adherence questionnaire assessed between baseline and 3.5 months | 6 sessions of family group therapy, with cartoon aids | 33 | NR | Baseline: 3.71 Follow-up: 4.81 | Intervention delivered following completion of study | 32 | NR | Baseline: 4.79 Follow-up: 4.36 | NR | 0.05 |
| Funck-Brentano [22] | Change in proportion of participants with undetectable HIV viral load (<200 copies/ml) between baseline and 24 months | Group 1: 90-min group therapy and peer support session once every 6 weeks for 26 months | 10 | 0 (0%) | Baseline: 3 (30%) Follow-up: 8 (80%) | Group 2: declined to participate | 10 | 1 (1%) | Baseline: 3 (33%) Follow-up: 5 (56%) | NR | NR |
| Group 3: lived too far to participate | 10 | 0 (0%) | Baseline: 5 (50%) Follow-up: 5 (50%) | NR | |||||||
NR, Not reported; CI, confidence interval; SE, standard error; DOT, directly observed therapy; OR, odds ratio.
Study included under linkage to ART and retention on ART.
Total of 3794 participants aged 10–14 years and 53 244 aged 15–24 years in the study as a whole.
Adjusted odds ratio.
Medication adherence score’ comprised of mean response to 3 items (‘per cent of days that any medications were taken, whether all doses were taken, and whether medication was taken according to instructions’).
Odds ratio for difference between groups in rate of change in mean medication adherence score.
Not clear what scores are measuring from manuscript.
Retention on ART
Two studies [20, 25] investigated the effect of interventions to improve adolescents’ retention on ART (Table 1b). Interventions evaluated included a multidisciplinary adolescent clinic staffed by adolescent physicians, care managers and social workers [20]; decentralisation of care (defined by authors to be no provision of specific adolescent-targeted services) [20]; peer support groups [20, 25]; and adolescent-specific clinic opening hours [20, 25].
In an ecological study in one US HIV clinic, adolescents who had access to multidisciplinary adolescent clinics with individualised support were significantly less likely to have gaps in care compared to adolescents treated when these services were not available [20]. Surprisingly, in evaluation of programmatic data from four sub-Saharan countries [25], the cumulative incidence of attrition from ART 1 year after starting treatment was significantly higher amongst clinic attenders where adolescent-specific clinic opening hours were available. Availability of peer support groups was associated with lower risk of attrition from ART in univariate analysis, but was not examined in adjusted models [25].
Adherence to ART
In total, five interventions to improve adolescents’ adherence to ART were evaluated, including counselling and education; use of adherence support devices; financial incentives; peer support; and directly observed therapy (Table 1c).
Seven studies evaluated counselling and education interventions [18, 19, 21, 22, 24, 26, 27]. Counselling and education were offered to adolescents and their carers by clinic staff and researchers in a variety of ways, including as individuals sessions with a single counsellor [18]; family sessions with a single counsellor [21]; multidisciplinary sessions with an adolescent and/or their carers [19, 26, 27]; and group counselling sessions [22, 24]. Some studies used established counselling curricula used in previous studies such as multisystemic therapy [26] and motivational interviewing [21], whilst others used open-ended and bespoke counselling and education techniques [18, 19, 22, 24, 27].
In studies of counselling and education, although participant groups, settings and content of interventions varied considerably, there was some evidence of effectiveness, although caution is required due to small study sizes and methodological issues. In a non-randomised study conducted amongst adolescents attending two HIV clinics in Roi-Et Province in Thailand, adolescents who received group counselling aimed at improving empowerment had greater improvements in ART adherence compared to adolescents who did not receive group counselling [24]. Adolescents with adherence difficulties attending an urban clinic in the USA who participated in 12-week multidisciplinary family counselling sessions showed substantial reductions in missed ART doses at the end of the programme [27].
Peer counselling (group counselling sessions where adolescents patients provided motivation and support to each, often facilitated by a clinic staff member or researcher) was investigated in one study of adolescents attending a hospital outpatient clinic in France [22]. Groups were not randomly allocated to receive peer support or control interventions and numbers were small. After 2 years of follow-up, the proportion of participants achieving viral suppression was higher amongst adolescents who received peer counselling.
In a study from a UK specialist centre, variable amounts of financial incentives linked to achievement of HIV viral load results were offered to adolescents with complex ART histories and previous multiple treatment interruptions [21]. Results were promising, but limited by small numbers and lack of comparison groups.
In two studies, adherence support devices (for example, medication boxes and beepers) were provided to individuals as part of packages of interventions, including educational and counselling [18], or motivational interviewing and financial incentives [21]. It is therefore difficult to distinguish the effects of adherence support devices from other interventions provided.
Two studies evaluated inpatient directly observed therapy (DOT) provided to adolescents with previous adherence difficulties or virological failure [23, 28]. Both studies had small numbers of participants, and although participants’ rates of virological failure showed improvements in the short term (by the end of completion of DOT), results were not sustained at 6 months after completing DOT.
Quality and risk of bias assessment
The methodological quality of included studies was assessed as weak (summarised in Table 2 and reported in detail for each study in Table S3). All three of the included randomised controlled trials had methodological and reporting factors that meant they were classified as being at low methodological quality. Notably, none of the trials completely followed the CONSORT statement guidelines for reporting randomised controlled trials. Particular concerns were noted with procedures for randomisation and allocation (either at high risk of bias or not reported) [18, 19, 26], protocol-driven consistency in application of interventions to participants [18, 19, 26] and selective reporting of outcomes [19, 26].
Table 2.
Methodological quality and risk of bias assessment for included studies
| Randomised controlled trials | |||||||
|---|---|---|---|---|---|---|---|
| Domains | Sequence generation |
Blinding of participants and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of potential bias | ||
| Signalling questions | Was the allocation sequence adequately generated? | Was allocation adequately concealed? | Was knowledge of the allocated intervention adequately prevented during the study? | Were incomplete outcome data adequately addressed? | Are reports of the study free of suggestion of selective outcome reporting | Was the study free of other problems that could put it at a high risk of bias? | Overall quality of study |
| Adolescents’ completion of steps of HIV care pathway | |||||||
| No studies | |||||||
| Adolescents’ retention on antiretroviral therapy | |||||||
| No studies | |||||||
| Adolescents’ adherence to antiretroviral therapy | |||||||
| Berrian [18] | Yes | No | No | Yes | Unclear | No | Low |
| Bhana [19] | Unclear | Unclear | Unclear | No | No | No | Low |
| Letourneau [26] | No | No | No | No | No | No | Low |
| Non-randomised studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant selection |
Exposure to intervention |
Comparability | Assessment of outcomes |
Other potential sources of bias | |||||||
| Domains | Were participants selected to be representative of the target population | Were there clear participant selection criteria (for both exposed and control group if relevant), avoiding in appropriate exclusions? | Was a comparator/control group assessed? | Was the intervention being studied applied consistently to all eligible participants? | Were potential confounders identified and appropriately adjusted for? | Were procedures for assessment of outcome sufficient to satisfy confirmation of presence of condition of interest? | Was follow-up long enough for outcome to occur? | Were incomplete outcome data adequately addressed? | Are reports of the study free of suggestion of selective outcome reporting? | Was the study free of other problems that could put it at a high risk of bias? | Overall quality of study |
| Adolescents’ completion of steps of HIV care pathway | |||||||||||
| Lamb [25] | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes | No | Good |
| Adolescents’ retention on antiretroviral therapy | |||||||||||
| Davila [20] | Unclear | Yes | Yes | No | No | Unclear | Yes | No | No | No | Good |
| Lamb [25] | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes | No | Good |
| Adolescents’ adherence to antiretroviral therapy | |||||||||||
| Foster [21] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Good |
| Funck-Brentano [22] | No | No | Yes | Yes | No | No | Yes | Unclear | No | No | Low |
| Glikman [23] | No | Yes | No | Unclear | No | Yes | Yes | Yes | No | No | Moderate |
| Kaihin [24] | Unclear | No | Yes | Unclear | No | No | Yes | Yes | Yes | No | Moderate |
| Lyon [27] | No | No | No | Unclear | No | Yes | Yes | Yes | Yes | No | Moderate |
| Parsons [28] | Yes | Yes | No | No | No | No | Yes | Yes | Yes | No | Moderate |
None of the randomised controlled trials reported calculations to determine optimal sample sizes for detection of effects, and all three sample sizes were small, ranging from 34 [26] to 65 [19] randomised participants. The relative or absolute effects of interventions were only reported by one randomised controlled trial [26], and no trials reported confidence intervals around effect estimates, with studies instead usually reporting only P-values for between-group comparisons. The possible effects of confounding variables were not adequately considered in any of the randomised controlled trials identified by the review. Handling of missing exposure and outcome data was also problematic in two studies [19, 26].
Of the eight non-randomised studies included, three studies were assessed as being of good methodological quality [20, 21, 25], four studies were of moderate quality [23, 24, 27, 28], and one was of low quality [22]. Common methodological problems included small sample sizes [20–24, 27, 28]; inconsistency in application of interventions to participants [20, 28]; failure to identify and appropriately adjust for potential confounding variables [20–24, 27, 28]; lack of comparison groups [20, 21, 23, 27, 28]; and selective outcome reporting [20, 22, 23]. In the studies reporting on interventions to improve adolescents’ adherence to ART, particular concerns included the use of non-standard and non-validated measures of adherence.
Discussion
The main findings from this systematic review were that, despite having worse HIV care outcomes than other age groups [1], few studies that have evaluated interventions to support adolescents’ linkage, retention and adherence to ART have been reported. As we move towards an era of universal treatment for HIV [10], the clinical and public health benefits of widening access to ART for adolescents will not be realised until cost-effective and sustainable service delivery interventions are widely implemented. The current evidence base from research on how best to do this is very weak. Although there are hints that some interventions may be effective, there is an urgent need for more rigorous, and larger, studies to clarify the effectiveness and replicability of potentially encouraging interventions.
We identified only 11 studies over a period of 13 and a half years, emphasising that the particular needs of adolescents living with HIV have not been widely studied [5]. Moreover, the majority of studies were conducted in the USA (n = 6) or Europe (n = 2), with only three studies conducted in countries with generalised HIV epidemics, where the majority of the world’s adolescents live. Most of the reports were of very small studies, with 8 of the 11 studies including fewer than 50 participants. The three randomised controlled trials were all small, with 34 [26], 37 [18] and 65 [19] participants, respectively. The only two studies with over 100 participants were based on a retrospective cohort study in USA with a total of 174 participants over the three eras of care that were compared [20], and a very large multicountry study that used routinely reported clinic data [25].
The service delivery interventions that were identified in this review were predominantly focused on relatively resource-intensive approaches applied at the individual level, such as motivational interviewing [26], counselling and peer support [19, 22, 24, 27], education [19], directly observed therapy [23, 28] and financial incentives [21]. This likely reflects attempts to overcome the psychological, behavioural and educational difficulties experienced by adolescents (which may be contributory to poor outcomes) [29]. However, interventions targeted at this level alone are unlikely to be generalisable or sufficient to overcome the considerable structural and health system barriers faced by adolescents. Complementary interventions, acting at different levels, are likely to be required. A recent systematic review of interventions to improve linkage from HIV diagnosis to initiation of ART amongst all HIV-infected people in low- and middle-income countries similarly identified a small number of studies, with most judged to be of poor quality, and the authors noted that few of the studies evaluated interventions specifically targeted to adolescents [30].
In contrast to the situation for adolescents, a greater number of interventions to improve adults’ linkage, retention and adherence to ART have been evaluated and have been introduced into routine clinical practice, especially in resource-limited settings [30, 31]. At the policy and health systems levels, task-shifting to lower cadres of health workers and non-medical personal, decentralising care to primary care level and home delivery of HIV care including ART initiation has increased retention in care and improved acceptability [32], whilst decentralising care to primary care level has improved accessibility [33]. Home initiation and delivery of HIV care have been shown to be effective in improving linkage and retention in care in well-powered cluster-randomised controlled trials [34, 35]. Integration of HIV care services into general medical clinics has been widely implemented [36]. At first glance, such interventions seem to be promising approaches that could be offered to adolescents. However, adolescents may have substantially different clinical, social and emotional needs than adults and unmodified implementation of interventions that have been designed for adults may not be appropriate. One example of an adolescent-tailored intervention identified in this review was ‘adolescent-friendly’ clinics, with special opening hours for adolescents and targeted services [25]. However, limitations in the study’s application of interventions and lack of clear evidence of effectiveness preclude recommendation of this strategy until further high-quality studies are conducted.
This study had important strengths, including the comprehensive search strategy, rigorous methods and wide scope covering all aspects of the HIV care pathway for adolescents who had been diagnosed with HIV. However, there were limitations. Of principal concern was the apparent low-to-moderate quality of the majority of studies, meaning that any policy recommendations for implementation must be made with extreme caution until further, high-quality studies have been conducted and adequately reported. Age definitions of adolescence varied widely between studies, and some studies that included adolescent participants were excluded because the majority of participants were not aged between 10 and 19 years, or we were unable to extract data for this age group [37–76]. In informal review of these studies, most of the interventions assessed were broadly similar to those included in the review and also had low methodological and/or reporting quality.
Because of the wide scope of the study and large numbers of possible study types included, it is possible that a small number of studies were inadvertently excluded because of our search or inclusion criteria. For example, due to time and logistic constraints, we only included studies published in English. This may partly explain the lack of studies from Latin America. In accordance with our published study protocol, we did not attempt to estimate summary measures of effectiveness for interventions due to the high degree of heterogeneity in the interventions identified.
Conclusions and recommendations
Over a period of 13.5 years, only 11 studies examining the effect of interventions on adolescents’ linkage, retention or adherence to ART were identified, with most studies conducted in high-income countries and using relatively resource-intensive, individual client approaches. Overall, the methodological quality of the reports of the identified studies was poor, and most studies had very few participants. Although qualified by the poor quality of studies, some approaches appear promising and need to be evaluated in larger, rigorous studies. These approaches, along with those identified in studies that had a combination of adults and adolescents [30, 32, 33, 77, 78], are summarised in Box 1.
Box 1. Interventions identified in this review that warrant further investigation.
- For all HIV infected adolescents:
-
○Improved accessibility to clinics and availability of youth-friendly services
-
○Multidisciplinary adolescent HIV clinics
-
○Peer counselling and support
-
○
- For adolescents experiencing treatment failure or poor adherence:
-
○Counselling (individual and family)
-
○Financial incentives
-
○Provision of adherence support devices (e.g. pill boxes and beepers)
-
○
Adolescents living with HIV are currently a neglected group. To ensure they are able to achieve the benefits of ART, rigorous evaluation of a wider range of existing and innovative service delivery interventions applied at the individual, health facility and policy levels are urgently required. When designing interventions, studies need to be cognisant of the limited resources available within health systems, which are particularly constrained in the countries with generalised HIV epidemics and large numbers of HIV-infected adolescents.
Supplementary Material
Figure S1. Search strategy.
Figure S2. PRISMA flow diagram.
Table S1. (a) Characteristics of studies reporting on interventions to improve adolescents’ linkage to ART. (b) Characteristics of studies reporting on interventions to improve adolescents’ retention on ART. (c) Characteristics of studies reporting on interventions to improve adolescents’ adherence to ART.
Table S2. Characteristics of excluded studies.
Table S3. Detailed risk of bias assessment for included studies.
Acknowledgments
Funding
This work was commissioned by the World Health Organization. The content is solely the responsibility of the authors and does not necessarily represent the official views of the World Health Organization.
Footnotes
Free full access from www.tmih.com
Additional Supporting Information may be found in the online version of this article:
References
- 1.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 2.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–851. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernays S, Jarrett P, Kranzer K, Ferrand RA. Children growing up with HIV infection: the responsibility of success. Lancet. 2014;383:1355–1357. doi: 10.1016/S0140-6736(13)62328-4. [DOI] [PubMed] [Google Scholar]
- 4.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 5.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–639. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowshen N, D’Angelo L. Health care transition for youth living with HIV/AIDS. Pediatrics. 2011;128:762–771. doi: 10.1542/peds.2011-0068. [DOI] [PubMed] [Google Scholar]
- 7.Philbin MM, Tanner AE, Duval A, et al. Factors affecting linkage to care and engagement in care for newly diagnosed HIV-positive adolescents within fifteen adolescent medicine clinics in the United States. AIDS Behav. 2013;18:1501–1510. doi: 10.1007/s10461-013-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MP, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008-2013. AIDS. 2015;29:493–502. doi: 10.1097/QAD.0000000000000559. (London, England) [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to anti-retroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28:1945–1956. doi: 10.1097/QAD.0000000000000316. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 11.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Priority Interventions. HIV/AIDS Prevention, Treatment and Care in the Health Sector. World Health Organization; Geneva: 2009. [Google Scholar]
- 14.World Health Organization . Retention in HIV Programmes: Defining the Challenges and Identifying Solutions. World Health Organization; Geneva: 2011. [Google Scholar]
- 15.MacPherson P, Munthali C, Ross D. Service delivery interventions to improve adolescents’ retention, adherence and linkage to HIV care: a systematic review. Protocol number PROSPERO International Prospective Register of Systematic Reviews. 2014 CRD42014012858 http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014012858#.VT325M6Qb8s.
- 16.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 18.Berrien VM, Salazar JC, Reynolds E, McKay K. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18:355–363. doi: 10.1089/1087291041444078. [DOI] [PubMed] [Google Scholar]
- 19.Bhana A, Mellins CA, Petersen I, et al. The VUKA family program: piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care. 2014;26:1–11. doi: 10.1080/09540121.2013.806770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, Giordano TP. Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care. 2013;25:202–206. doi: 10.1080/09540121.2012.689811. [DOI] [PubMed] [Google Scholar]
- 21.Foster C, McDonald S, Frize G, Ayers S, Fidler S. “Payment by Results”–financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS. 2014;28:28–32. doi: 10.1089/apc.2013.0262. [DOI] [PubMed] [Google Scholar]
- 22.Funck-Brentano I, Dalban C, Veber F, et al. Evaluation of a peer support group therapy for HIV-infected adolescents. AIDS. 2005;19:1501–1508. doi: 10.1097/01.aids.0000183124.86335.0a. (London, England) [DOI] [PubMed] [Google Scholar]
- 23.Glikman D, Walsh L, Valkenburg J, Mangat PD, Marcinak JF. Hospital-based directly observed therapy for HIV-infected children and adolescents to assess adherence to anti-retroviral medications. Pediatrics. 2007;119:e1142–e1148. doi: 10.1542/peds.2006-2614. [DOI] [PubMed] [Google Scholar]
- 24.Kaihin R, Kasatpibal N, Chitreechuer J, Grimes RM. Effect of an empowerment intervention on antiretroviral drug adherence in Thai Youth. Behav Med. 2014;23:1–9. doi: 10.1080/08964289.2014.911717. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15-24 years of age) enrolled in HIV care. AIDS. 2014;28:559–568. doi: 10.1097/QAD.0000000000000054. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letourneau EJ, Ellis DA, Naar-King S, Chapman JE, Cunningham PB, Fowler S. Multisystemic therapy for poorly adherent youth with HIV: results from a pilot randomized controlled trial. AIDS Care. 2013;25:507–514. doi: 10.1080/09540121.2012.715134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyon ME, Trexler C, Akpan-Townsend C, et al. A family group approach to increasing adherence to therapy in HIV-infected youths: results of a pilot project. AIDS Patient Care STDS. 2003;17:299–308. doi: 10.1089/108729103322108175. [DOI] [PubMed] [Google Scholar]
- 28.Parsons GN, Siberry GK, Parsons JK, et al. Multidisciplinary, inpatient directly observed therapy for HIV-1-infected children and adolescents failing HAART: a retrospective study. AIDS Patient Care STDS. 2006;20:275–284. doi: 10.1089/apc.2006.20.275. [DOI] [PubMed] [Google Scholar]
- 29.Kasedde S, Luo C, McClure C, Chandan U. Reducing HIV and AIDS in adolescents: opportunities and challenges. Curr HIV/AIDS Rep. 2013;10:159–168. doi: 10.1007/s11904-013-0159-7. [DOI] [PubMed] [Google Scholar]
- 30.Govindasamy D, Meghij J, Kebede Negussi E, Clare Baggaley R, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings–a systematic review. J Int AIDS Soc. 2014;17:19032. doi: 10.7448/IAS.17.1.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. W-284, W-5, W-6, W-7, W-8, W-9, W-90, W-91, W-92, W-93, W-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kredo T, Adeniyi FB, Bateganya M, Pienaar ED. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. Cochrane Database Syst Rev. 2014;7:CD007331. doi: 10.1002/14651858.CD007331.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kredo T, Ford N, Adeniyi FB, Garner P. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Database Syst Rev. 2013;6:CD009987. doi: 10.1002/14651858.CD009987.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacPherson P, Lalloo DG, Webb EL, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. JAMA. 2014;312:372–379. doi: 10.1001/jama.2014.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwarenstein M, Fairall LR, Lombard C, et al. Outreach education for integration of HIV/AIDS care, antiretroviral treatment, and tuberculosis care in primary care clinics in South Africa: PALSA PLUS pragmatic cluster randomised trial. BMJ. 2011;342:d2022. doi: 10.1136/bmj.d2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basso CR, Helena ETS, Caraciolo JMM, Paiva V, Nemes MIB. Exploring ART intake scenes in a human rights-based intervention to improve adherence: a randomized controlled trial. AIDS Behav. 2013;17:181–192. doi: 10.1007/s10461-012-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braitstein P, Siika A, Hogan J, et al. A clinician-nurse model to reduce early mortality and increase clinic retention among high-risk HIV-infected patients initiating combination anti-retroviral treatment. J Int AIDS Soc. 2012;15:7. doi: 10.1186/1758-2652-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung AM, Mancao N. A pharmacist-managed medication adherence program to improve antiretroviral therapy adherence and clinical outcomes in HIV/AIDS-infected adults and children. Pharmacotherapy. 2005;25:479. [Google Scholar]
- 40.de Bruin M, Hospers HJ, van Breukelen GJ, Kok G, Koevoets WM, Prins JM. Electronic monitoring-based counseling to enhance adherence among HIV-infected patients: a randomized controlled trial. Health Psychol. 2010;29:421–428. doi: 10.1037/a0020335. [DOI] [PubMed] [Google Scholar]
- 41.Dieckhaus KD, Odesina V. Outcomes of a multifaceted medication adherence intervention for HIV-positive patients. AIDS Patient Care STDS. 2007;21:81–91. doi: 10.1089/apc.2006.0044. [DOI] [PubMed] [Google Scholar]
- 42.DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003;14:52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 43.Dowshen N, Kuhns L, Johnson A, Holoyda B, Garofalo R. Text message reminders to improve adherence to antiretroviral therapy for HIV-positive youth. J Adolesc Health. 2011;48:S64–S65. [Google Scholar]
- 44.Dowshen N, Kuhns LM, Gray C, Lee S, Garofalo R. Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+ youth. AIDS Behav. 2013;17:2237–2243. doi: 10.1007/s10461-013-0464-6. [DOI] [PubMed] [Google Scholar]
- 45.Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Internet Res. 2012;14:e51. doi: 10.2196/jmir.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fatti G, Shaikh N, Eley B, Grimwood A. Improved virological suppression in children on antiretroviral treatment receiving community-based adherence support: a multicentre cohort study from South Africa. AIDS Care. 2014;26:448–453. doi: 10.1080/09540121.2013.855699. [DOI] [PubMed] [Google Scholar]
- 47.Finocchario-Kessler S, Catley D, Thomson D, Bradley-Ewing A, Berkley-Patton J, Goggin K. Patient communication tools to enhance ART adherence counseling in low and high resource settings. Patient Educ Couns. 2012;89:163–170. doi: 10.1016/j.pec.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. (London, England) [DOI] [PubMed] [Google Scholar]
- 49.Garvie PA, Lensing S, Rai SN. Efficacy of a pill-swallowing training intervention to improve antiretroviral medication adherence in pediatric patients with HIV/AIDS. Pediatrics. 2007;119:e893–e899. doi: 10.1542/peds.2006-1488. [DOI] [PubMed] [Google Scholar]
- 50.Gaur AH, Belzer M, Britto P, et al. Directly observed therapy (DOT) for nonadherent HIV-infected youth: lessons learned, challenges ahead. AIDS Res Hum Retroviruses. 2010;26:947–953. doi: 10.1089/aid.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giordano TP, Rodriguez S, Zhang H, et al. Effect of a clinic-wide social marketing campaign to improve adherence to antiretroviral therapy for HIV infection. AIDS Behav. 2013;17:104–112. doi: 10.1007/s10461-012-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goggin K, Gerkovich MM, Williams KB, et al. A randomized controlled trial examining the efficacy of motivational counseling with observed therapy for antiretroviral therapy adherence. AIDS Behav. 2013;17:1992–2001. doi: 10.1007/s10461-013-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haberer JE, Kiwanuka J, Nansera D, et al. Realtime adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS. 2013;27:2166–2168. doi: 10.1097/QAD.0b013e328363b53f. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igumbor JO, Scheepers E, Ebrahim R, Jason A, Grimwood A. An evaluation of the impact of a community-based adherence support programme on ART outcomes in selected government HIV treatment sites in South Africa. AIDS Care. 2011;23:231–236. doi: 10.1080/09540121.2010.498909. [DOI] [PubMed] [Google Scholar]
- 55.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 56.Kamau TM, Olsen VG, Zipp GP, Clark M. The effectiveness of social resource intervention to promote adherence to HIV medication in a multidisciplinary care setting in Kenya. Int J STD AIDS. 2012;23:843–848. doi: 10.1258/ijsa.2012.011472. [DOI] [PubMed] [Google Scholar]
- 57.Kenya S, Jones J, Arheart K, et al. Using community health workers to improve clinical outcomes among people living with HIV: a randomized controlled trial. AIDS Behav. 2013;17:2927–2934. doi: 10.1007/s10461-013-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunutsor S, Walley J, Katabira E, et al. Using mobile phones to improve clinic attendance amongst an antiretroviral treatment cohort in rural Uganda: a cross-sectional and prospective study. AIDS Behav. 2010;14:1347–1352. doi: 10.1007/s10461-010-9780-2. [DOI] [PubMed] [Google Scholar]
- 59.Kunutsor S, Walley J, Muchuro S, et al. Improving adherence to antiretroviral therapy in sub-Saharan African HIV-positive populations: an enhanced adherence package. AIDS Care. 2012;24:1308–1315. doi: 10.1080/09540121.2012.661833. [DOI] [PubMed] [Google Scholar]
- 60.Magnano San Lio M, Mancinelli S, Palombi L, et al. The DREAM model’s effectiveness in health promotion of AIDS patients in Africa. Health Promot Int. 2009;24:6–15. doi: 10.1093/heapro/dan043. [DOI] [PubMed] [Google Scholar]
- 61.Magnus M, Jones K, Phillips G, 2nd, et al. Characteristics associated with retention among African American and Latino adolescent HIV-positive men: results from the outreach, care, and prevention to engage HIV-seropositive young MSM of color special project of national significance initiative. J Acquir Immune Defic Syndr. 2010;53:529–536. doi: 10.1097/QAI.0b013e3181b56404. [DOI] [PubMed] [Google Scholar]
- 62.Patten GE, Wilkinson L, Conradie K, et al. Impact on ART initiation of point-of-care CD4 testing at HIV diagnosis among HIV-positive youth in Khayelitsha, South Africa. J Int AIDS Soc. 2013;16:18518. doi: 10.7448/IAS.16.1.18518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puccio JA, Belzer M, Olson J, et al. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. AIDS Patient Care STDS. 2006;20:438–444. doi: 10.1089/apc.2006.20.438. [DOI] [PubMed] [Google Scholar]
- 64.Rawlings MK, Thompson MA, Farthing CF, et al. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34:174–183. doi: 10.1097/00126334-200310010-00007. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds E, Berrien V, Acosta-Glynn C, Salazar JC. Home based, intense, nursing intervention trial improves adherence to HAART in HIV infected children. Pediatric Academic Societies Annual Meeting; Baltimore, MD. 2001. [Google Scholar]
- 66.Rongkavilit C, Naar-King S, Koken JA, et al. A feasibility study of motivational interviewing for health risk behaviors among Thai youth living with HIV. J Assoc Nurses AIDS Care. 2014;25:92–97. doi: 10.1016/j.jana.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rotheram-Borus MJ, Swendeman D, Comulada WS, Weiss RE, Lee M, Lightfoot M. Prevention for substance-using HIV-positive young people: telephone and in-person delivery. J Acquir Immune Defic Syndr. 2004;37(Suppl 2):S68–S77. doi: 10.1097/01.qai.0000140604.57478.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simoni JM, Huh D, Frick PA, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52:465–473. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snyder K, Wallace M, Duby Z, et al. Preliminary results from Hlanganani (Coming Together): a structured support group for HIV-infected adolescents piloted in Cape Town, South Africa. Child and Youth Serv Rev. 2014;45:114–121. [Google Scholar]
- 70.Van Winghem J, Telfer B, Reid T, et al. Implementation of a comprehensive program including psycho-social and treatment literacy activities to improve adherence to HIV care and treatment for a pediatric population in Kenya. BMC Pediatr. 2008;8:52. doi: 10.1186/1471-2431-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belzer M. A pilot study using cell phone interactions to improve HIV medication adherence in adolescents who have previously failed antiretroviral therapy. J Adolesc Health. 2013;52:S7. [Google Scholar]
- 72.Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: finding, linking, and retaining young HIV-positive black and Latino men who have sex with men in care. AIDS Patient Care STDS. 2011;25:37–45. doi: 10.1089/apc.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naar-King S, Outlaw AY, Sarr M, et al. Motivational Enhancement System for Adherence (MESA): pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol. 2013;38:638–648. doi: 10.1093/jpepsy/jss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naar-King S, Parsons JT, Murphy DA, Chen X, Harris DR, Belzer ME. Improving health outcomes for youth living with the human immunodeficiency virus: a multisite randomized trial of a motivational intervention targeting multiple risk behaviors. Arch Pediatr Adolesc Med. 2009;163:1092–1098. doi: 10.1001/archpediatrics.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saberi P, Mayer K, Vittinghoff E, Naar-King S. Correlation Between Use of Antiretroviral Adherence Devices by HIV-Infected Youth and Plasma HIV RNA and Self-Reported Adherence. AIDS Behav. 2014;19:93–103. doi: 10.1007/s10461-014-0806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wohl AR, Garland WH, Wu J, et al. A youth-focused case management intervention to engage and retain young gay men of color in HIV care. AIDS Care. 2011;23:988–997. doi: 10.1080/09540121.2010.542125. [DOI] [PubMed] [Google Scholar]
- 77.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;3:CD009756. doi: 10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17:18809. doi: 10.7448/IAS.17.1.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Search strategy.
Figure S2. PRISMA flow diagram.
Table S1. (a) Characteristics of studies reporting on interventions to improve adolescents’ linkage to ART. (b) Characteristics of studies reporting on interventions to improve adolescents’ retention on ART. (c) Characteristics of studies reporting on interventions to improve adolescents’ adherence to ART.
Table S2. Characteristics of excluded studies.
Table S3. Detailed risk of bias assessment for included studies.