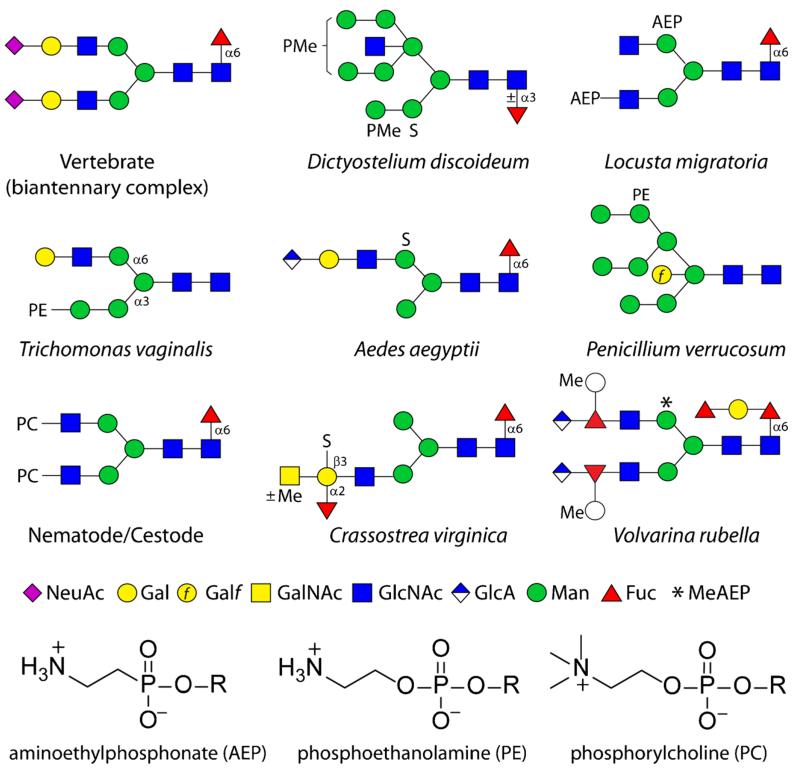
Fig. 1.
Example anionic and zwitterionic N-glycans from non-mammalian sources. Glycans are shown according to the nomenclature of the Consortium for Functional Glycomics [11] as compared to a biantennary sialylated vertebrate N-glycan (all sugars are pyranose, except for Galf, galactofuranose). AEP, aminoethylphosphonate; PC, phosphorylcholine; PE, phosphoethanolamine; PMe, methylphosphate; S, sulphate; *, N-methylaminoethylphosphonate. The chemical structures of the zwitterionic modifications are also shown. The named organisms in which these example glycans are found may not represent the only species to contain these structures, but are the ones in which these are proven [4, 12–19]