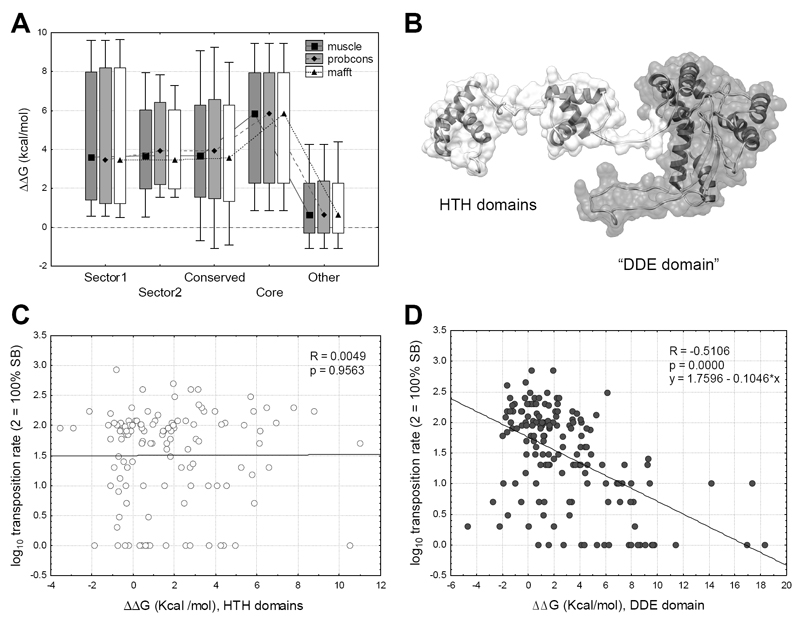
Figure 4. The effect of mutations on the change of the free energy of unfolding.
(median, box: 25%-75%, whiskers: 10%-90%). A) Mutations in sectors and the core are significantly more destabilizing (ΔΔG > 0) than mutants of other residues (p < 0.05 for all comparisons, t-tests). B) The monomer of SB. The flexible N-terminal arm of the protein containing the HTH domains (residues 1-120) is indicated with white, the globular part (residues 121-340), which contains the DDE domain, with gray. C) In the flexible arm the effect of mutations on ΔΔG is not correlated with transposition rate (p = 0.95). D) In the globular region we find a significant negative correlation (p << 0.001, R = -0.51) between ΔΔG and transposition rate.