Figure 1.
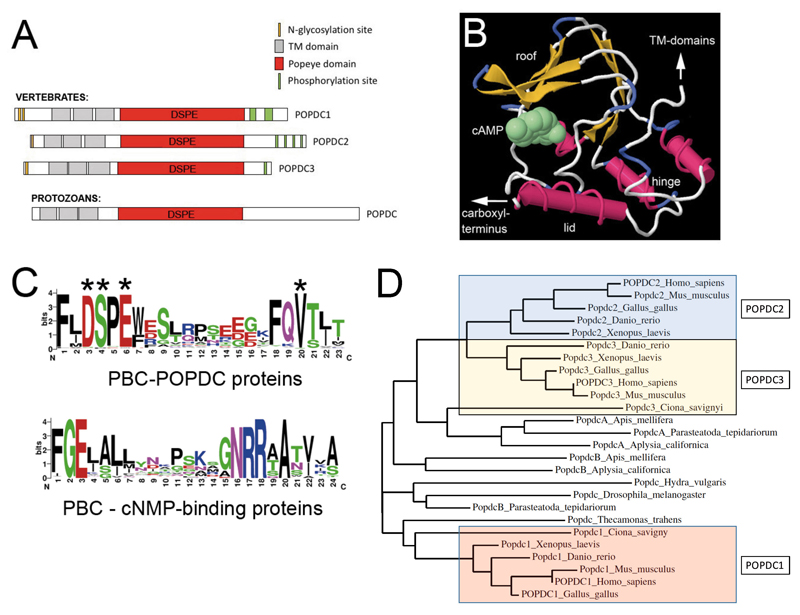
The Popeye domain containing protein family. (A) Graphic depiction of the domain structure of POPDC proteins. POPDC proteins consist of a short extracellular amino terminus, which is subject to N-glycosylation, followed by three transmembrane domains and the Popeye domain. The approximate location of the invariant DSPE motif, which is part of the phosphate-binding cassette (PBC) is indicated. The carboxy terminal part of the proteins is variable in size, differs between family members, and contain cluster of serine/threonine residues, which are subject to phosphorylation in response to β-adrenergic stimulation [16]. The structure of a protozoan POPDC protein has an overall identical structure, suggesting an ancient origin of the POPDC protein family. (B) Structural model of the Popeye domain of POPDC1. The 3-D model depicted here was generated using the First Glance in Jmol website [17]. The structural elements (roof, hinge and lid) of the Popeye domain have been identified based on their similar location in other cAMP-binding domains [6]. (C) Comparison of the PBC of POPDC proteins and that of canonical cyclic nucleotide monophosphate (cNMP)-binding proteins. In POPDC proteins cAMP-binding involves the ultra-conserved sequence motifs DSPE and FQVT. Experimental evidence for the involvement in cAMP-binding has been obtained for D200, S201, E203, and V217 (asterisks). The canonical cAMP-binding cassette strongly diverges from the one present in POPDC proteins. Sequence data utilized for the generation of the logo for the Popeye domain were retrieved from the PFAM entry of the Popeye domain [18]. For the protein sequences of non-POPDC cAMP binding proteins, the data were downloaded from Prosite (PS00889; CNMP_BINDING_2) [19,20]. (D) A phylogenetic tree of vertebrate and invertebrate POPDC protein sequences. The vertebrate Popdc proteins cluster into three groups: POPDC1, POPDC2 and POPDC3. The POPDC1 and POPDC3 proteins of the urochordate Ciona savigny appear to be orthologous to their respective vertebrate counterparts, supporting a model of two gene duplication events during chordate and vertebrate evolution, respectively. Popdc proteins in other invertebrates do show similarity to either Popdc1 or Popdc2/3 and thus protein ancestry cannot be easily deduced from this analysis. The tree was generated with the help of the Phylogeny online platform [21].