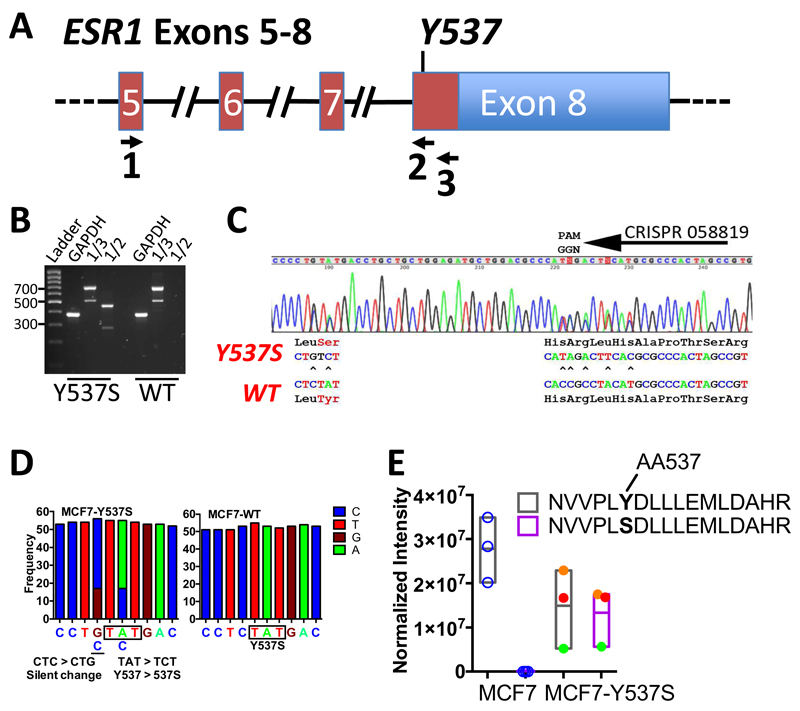
Figure 1. CRISPR-Cas9 directed generation of the Y537S mutation in the ESR1 gene in MCF7 breast cancer cells.
(A) Schematic representation of the ESR1 gene, exons 5-8, annotated for the positions of PCR primers used for RT-PCR analysis. (B) RT-PCR of MCF7 (WT) and MCF7-Y537S cell lines using primers in A. Expression of wild-type and mutant ER alleles was confirmed by RT-PCR, using primers in ESR1 Exon 5 (Primer 1, 5’-CCAGGGAAGCTACTGTTTGC-3’) and Exon 8 (Primer 3, 5’-GATGCATGCCGGAGTGTATG-3’), which generate a 700bp product. The ESR1 transcript arising from the Y537S mutant allele was amplified as a 466bp product using the exon 5 primer and the knockin specific primer. (Primer 2, 5’-GATGCATGCCGGAGTGTATG-3’). (C) Sequencing chromatogram of the RT-PCR products for the exon 8 coding region for MCF7-Y537S cells, showing expression of both mutant and wild-type alleles. (D) Frequency of RNA-seq reads for the Y537 and 537S codons in MCF7 and MCF7-Y537S cells. (E) Normalized intensity of ER tryptic peptide containing amino acid 537 from LC-MS. Results are shown for three independent immunoprecipitated ER samples for MCF7 and MCF7-Y537S lines. No signal was obtained for the mutant peptide in MCF7 cells. For the MCF7-Y537S samples, the normalised intensity for each replicate sample is shown by circles of the same colour, demonstrating similar amounts of the two peptides in MCF7-Y537S cells.