Abstract
Objective
To create a model of atherosclerosis using GFP-targeted monocytes/macrophages, allowing analysis of both endogenous GFP+ and adoptively transferred GFP+ myeloid cells in arterial inflammation.
Approach and Results
hCD68GFP reporter mice were crossed with ApoE−/− mice. Expression of GFP was localized to macrophages in atherosclerotic plaques and in Angiotensin II-induced aortic aneurysms, and correlated with Galectin 3 and mCD68 expression. Flow cytometry confirmed GFP+ expression in CD11b+/CD64+, CD11c+/MHC-IIHI and CD11b+/F4/80+ myeloid cells. Adoptive transfer of GFP+ monocytes demonstrated monocyte recruitment to both adventitia and atherosclerotic plaque, throughout the aortic root, within 72 hours. We demonstrated the biological utility of hCD68GFP monocytes by comparing the recruitment of wild-type and CCR2−/− monocytes to sites of inflammation.
Conclusions
hCD68GFP/ApoE−/− mice provide a new approach to study macrophage accumulation in atherosclerotic plaque progression, and to identify cells recruited from adoptively transferred monocytes.
Keywords: atherosclerosis, monocyte, macrophage, trafficking, mouse, model, GFP
Introduction
Monocyte/macrophage biology is a key driver of atherosclerotic disease 1. Recent observations highlight the importance of the origin and proliferation of macrophages within diseased arteries, and alterations in macrophage biology mediate plaque regression 2–4. The recently described hCD68GFP reporter mouse has abundant and persistent GFP expression in both circulating monocytes and tissue macrophages5. Because atherosclerosis is a complex vascular disease, with disease development occurring in multiple locations and analysis being heavily reliant of imaging or fluorescence technologies (histology, microscopy and flow cytometry), we hypothesized that high-level GFP expression within monocyte/macrophage populations would make the hCD68GFP mouse a powerful tool for probing myeloid cell biology in atherosclerotic plaque progression.
Materials and Methods
We used hCD68GFP mice5, which we have made available via Jackson Labs (Bar Harbor ME, USA), and ApoE−/− mice (Charles River UK Ltd) on C57Bl/6 background. Details of Methods can be found in the online-only Data Supplement.
Results
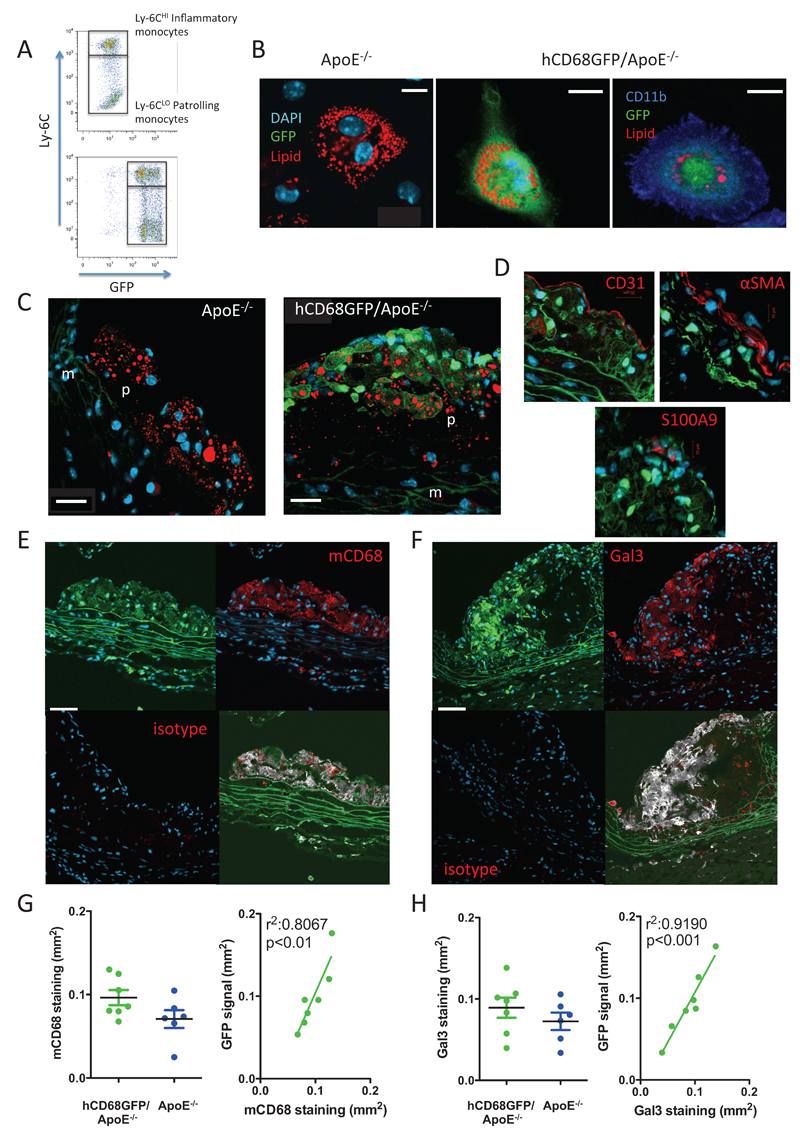
We crossed hCD68GFP mice with ApoE−/− mice to produce a line of homozygous ApoE−/− mice carrying a single copy of the hCD68-GFP locus. Expression of GFP was present in both Ly-6CHI and LO blood monocytes, as is the case in normolipidemic mice (Fig 1A). As GFP requires an aqueous environment for fluorescence, we confirmed a clear signal could also be seen in lipid-laden foam cells (Fig 1B). Exclusion of GFP from the lipid droplets was clear, but the remaining cytosol provided a bright fluorescent transgene signal. Lipid-laden cells were confirmed as macrophage-derived foam cells by co-expression of CD11b. We next examined the fluorescence of cells within aortic root plaque from animals fed a high fat diet for 10 weeks. GFP fluorescence was clearly visible within aortic root lesions, associated with areas of lipid accumulation (Fig 1C). This signal co-localised at a cellular level with the macrophage markers mCD68 and Galectin 3 (mac2 epitope), but not other plaque components (Fig 1D-F), using a thresholded co-localisation analysis. Analysis of the co-localisation in the luminal plaque showed a Mander’s overlap coefficient of M1: 0.923 and M2: 0.978, indicating a good coincidence of the green and red channel over the total intensity. Of note at a cellular level cells with the highest GFP expression did not necessarily express the highest levels of the macrophage markers, indicating that GFP expression is heterogeneous within the myeloid populations within the plaque. No co-localisation with GFP fluorescence was seen with CD31, S100A9 or alpha smooth muscle actin, to identify endothelial cells, neutrophils or smooth muscle cells within plaque (Fig 1D), with GFP threshold fluorescence set at the same intensities, although this does not exclude a low level fluorescence in other cell populations, as has been previously reported in neutrophils 5.
Figure 1. hCD68GFP/ApoE-/- mice show abundant GFP expression in arterial lesions.
A, Blood samples were harvested and stained to identify CD45+/CD115+/Ly6G- monocytes of both the Ly6CHI and LO phenotype. Compared to ApoE-/- mice the hCD68GFP/ApoE-/- mice have a clear increase in GFP fluorescence in both monocyte populations. B, 2% Thioglycollate solution was ip injected into mice that had been on a high fat diet for at least 8 weeks. 4 days following injection the elicited peritoneal cells were harvested and macrophages selected by adhesion to glass coverslips. Cells were fixed and stained with Lipidtox Red (red) to identify neutral lipid droplets and DAPI to visualize nuclei (blue), with cells being confirmed as macrophages by cell surface staining for CD11b expression (Right hand panel blue) (scale bar – 10μm). C, The aortic root from mice that had been maintained on a high fat diet for 10 weeks was fixed with 4% formaldehyde and cut into 7µm frozen sections. Staining for neutral lipids (red) revealed areas of strong GFP fluorescence (green) localized to lipid containing plaques only from mice harboring the hCD68GFP transgene, compared to ApoE-/- controls (p – plaque, m – media, DAPI - blue, scale bar – 20µm). D, Bright GFP fluorescence did not co-localise with markers of endothelial cells (CD31), infiltrating smooth muscle cells (alpha-smooth muscle actin) or neutrophils (S100A9). E and F, Strong co-localisation of GFP (green) at the cellular level was seen when sections were co-stained with anti-macrophage antibodies (targeted against mCD68 or Galectin 3) (red) and DAPI (blue) (scale bar – 20µm). A lack of staining in isotype control sections is shown in the lower left panel. Colocalisation is shown (grey) in the lower right panel, overlap with DAPI was removed to prevent any spillover, then pixels were assigned as green and/or red or neither using a threshold set on the non-GFP expressing/unstained controls. Pixels that were assigned both green and red were coloured grey in the resulting image. G and H, Quantification of the area of mCD68 and Gal3 staining within plaque from hCD68GFP/ApoE-/- and ApoE-/- mice showed no significant effect of GFP expression on macrophage infiltration into plaque and a highly significant correlation between GFP and mCD68/Gal3 staining. n=6-7, Students T-Test, p<0.05 regarded significant. . Linear regression analysis detected a highly significant relationship between GFP and mCD68/Gal3, p<0.05.
We next performed quantitative analysis of the GFP signal vs. mCD68 and Gal3 staining using fluorescence microscopy. We observed a strong correlation between the GFP fluorescence and macrophage markers; plaque macrophage content was not significantly affected by the hCD68GFP transgene (Fig1G-H). Additionally, there was no significant difference in macrophage infiltration in the aortic root plaques between GFP expressing and non-expressing littermates or in total plaque area, as measured using Masson’s Trichrome stain (Figure 1G-H and Supplementary Figure IA-B). A similar correlation between macrophage content and GFP fluorescence, with a lack of effect on total or macrophage plaque area was also observed in smaller plaques found in ApoE−/− animals fed a chow diet for 16 weeks (Supplementary Figure 1A-C). There was no confounding effect of hCD68-GFP on plasma lipid levels (HFD: Total cholesterol 41.2mmol/l (+/-2.01) (GFP+) vs 40.3 mmol/l (+/-3.44) (GFP-) HDL 2.4mmol/l (+/- 0.09) (GFP+) vs 2.4mmol/l (+/- 0.08) (GFP-); Chow: Total cholesterol 6.9mmol/l (+/- 1.3) (GFP+) vs 6.5mmol/l (+/- 0.8) (GFP-) HDL 1.27mmol/l (+/- 0.1) (GFP+) vs 1.33mmol/l (+/- 0.3) (GFP-)) .
The brightness of GFP expression in monocyte/macrophages from the hCD68GFP/ApoE−/− mouse enabled whole mount en-face analysis of the aorta, revealing plaque macrophages above the autofluorescence of the elastic lamina (Supplementary Figure II A-C). To identify these GFP+ foam cells, en-face preparations were permeabilised and stained with a mCD68 antibody, demonstrating that the large GFP+ cells present were macrophages (Supplementary Figure IIA). A stacked image revealed typical orientation of the CD31+ endothelial cell layer in the non-atherosclerosis prone outer curvature of the aortic arch (Supplementary Figure IIB). In contrast, imaging the inner curvature revealed a highly disorganized endothelium, with large clusters of GFP+ foam cells. We reconstructed images of the entire vessel wall, revealing that the GFP fluorescence was sufficiently bright to detect the accumulation of adventitial macrophages, adjacent to sites of arterial inflammation (Supplementary Figure IIC and Supplementary video file). The brightness of the transgene GFP signal will enable new studies of the crosstalk between luminal and adventitial inflammation in atherogenesis.
The infusion of angiotensin II into hCD68GFP/ApoE−/− mice caused a rapid recruitment of GFP+ myeloid cells to the aortic wall within 5 days (Supplementary Figure III). Prolonged infusion of angiotensin II caused aneurysm formation with abundant GFP+ cells in the vessel adventitia and hematoma border that co-express mCD68 (Supplementary Figure III).
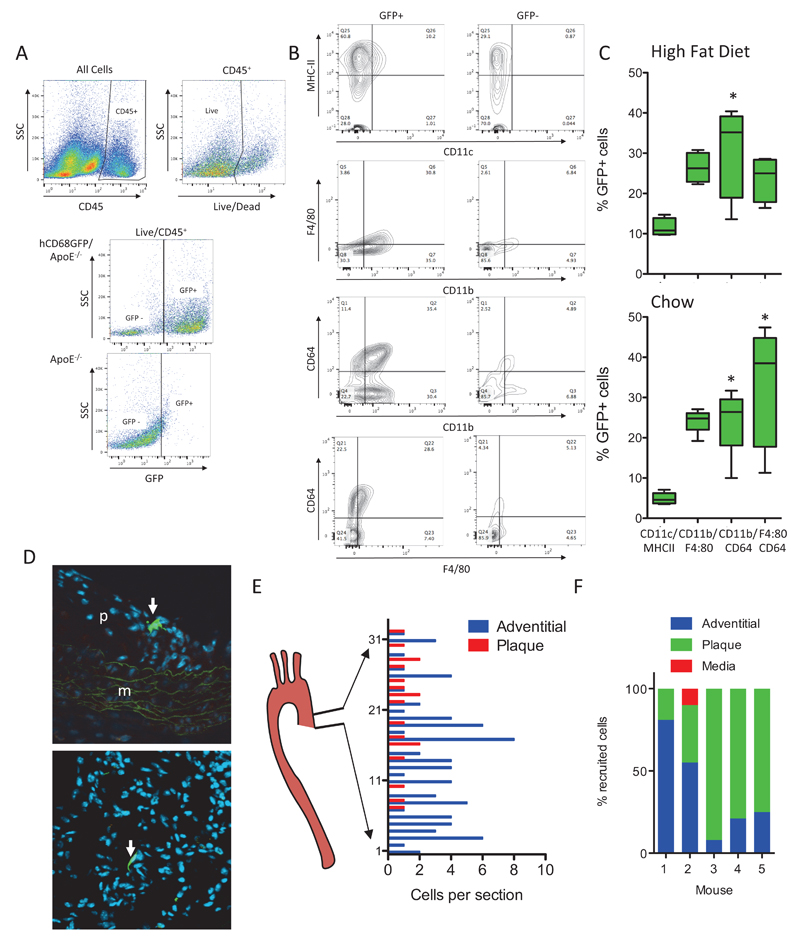
Whilst analysis of tissue sections is a mainstay of atherosclerotic analysis, detailed phenotyping of the cell populations in atherosclerotic plaques requires flow cytomtery, following tissue digests. We used a panel of antibodies that differentiate key myeloid cell populations to characterize in more detail the GFP+ cell types within the vessel wall. Interrogation of the live CD45+ population revealed that typically 54% (chow) to 60% (high fat fed) of the total leukocyte population in ApoE−/− aortae are GFP+ (Fig 2A and data not shown). When the GFP+ or GFP- CD45+ cells present were analysed within each hCD68GFP-ApoE-/- animal it was clear that the majority of macrophage populations were present within the GFP+ population, including those expressing markers CD11b+/CD64+, and the CD11b+/F4/80+ and CD11c+/MHCIIHI sub populations (Fig 2A-C). These GFP+ cell populations were found in both chow fed and high fat diet fed animals (Fig 2A-B and Supplementary Figure IV). Monocytes present within the aorta were also GFP+, as shown in aortic digests from angiotensin II infused mice (Supplementary Figure IV).
Figure 2. hCD68GFP/ApoE-/- mice enable identification of multiple myeloid populations in aortic lesions and allow tracking of adoptively transferred monocytes.
Aortic Digests demonstrate the presence of GFP expression in multiple myeloid populations within the descending aorta from mice fed a high fat diet for 10 weeks and harvested at 24weeks of age (female). Aortas were digested using a standard digest mixture (collagenase I, collagenase XI, hyaluronidase and DNAse I) with the resulting single cell suspension being stained with a viability dye and antibody cocktail to identify macrophage/dendritic cell populations (CD45, CD11b, CD64, CD11c, MHC-II, F4/80) by comparison to isotype control samples or GFP- controls. A, Viable leukocytes were identified as CD45+/live cells and GFP+ cells were gated by comparison to GFP- samples. B, Both GFP- and GFP+ populations within the Live/CD45 population were gated to identify the presence of myeloid cell populations within these two populations. The MHC-II/CD11c, CD11b/F4/80 and CD64/CD11b populations were found primarily within the GFP+ population. C, The relative contribution of the 3 populations to the total GFP+ population in aortas from both high fat fed and chow fed (16 week female mice – see Supplementary Figure 4) mice were quantified (one-way ANOVA with Dunn’s Multiple Comparison post test * = p<0.05 vs CD11c/MHC-II group, all other pairwise comparisons not significantly different; box and whisker plot max-min, n=4-5). D, 1.5x106 GFP+ monocytes isolated from bone marrow by negative selection were injected iv into mice that had been maintained on a high fat diet for 8 weeks. Tissue samples were harvested 72hrs later and the aortic root cut into frozen sections and stained with DAPI (blue) and GFP+ cells (green) visualized and counted in every second slide (GFP+ cells highlighted by white arrows). E, The number of GFP+ cells present per complete section analysed throughout out the aortic root from heart-aorta was plotted. F, Representative data from 5 adoptive transfer recipients demonstrated recruitment to both plaque and adventitial sites.
A major application of the hCD68GFP reporter mouse is the ability to track cells after adoptive transfer 5. Given the very high signal-to-background GFP fluorescence, we reasoned that adoptive transfer of monocytes into ApoE-/- mice would allow robust identification of individual monocytes migrating into atherosclerotic plaque. We performed monocyte isolation by monocyte enrichment using ‘no touch’ antibody depletion of other cell types from blood, bone marrow and spleen, selecting bone marrow as the source yielding the greatest number and purity of monocytes (Supplementary Figure V). Adoptive transfer of bone marrow monocytes, into high fat fed animals with established disease, was performed 72 hours prior to harvest. Analysis of sections throughout the entire aortic root revealed that GFP+ cells could be clearly visualized within the aortic root (Fig 2D). Adoptively transferred cells were observed both in the adventitia, where the cells had an elongated morphology with minimal protrusions, and within plaques, where cells had a spread morphology with multiple protrusions. Systematic quantification of the location of adoptively transferred monocytes revealed recruited cells in both the adventitia and the plaque throughout the aortic root, with recruitment to plaque being more prominent in the upper portion of the root (Fig 2E). The relative predominance of recruitment to plaque or adventitia varied between animals, indicating this model may be useful in determining the factors that control and influence sites of monocyte recruitment using laser capture microscopy or recovery of GFP+ cells by FACS (Fig 2F).
In addition to allowing the study of these rare cells, we also tested the utility of adoptive monocyte transfer into acute inflammation models such as sterile peritonitis, with quantification of genotype-specific effects on the donor cells. Although CCR2-/- mice have significantly reduced proportions of both Ly6CHI and Ly6CLO monocytes 6, isolation of bone marrow monocyte yielded cell samples with similar proportions of Ly6CHI monocytes (30% vs 25.5%), enabling us to directly compare recruitment of these cells in wildtype CCR2 replete host animals (Supplementary Figure VI). We compared transfer of hCD68GFP vs. hCD68GFP/CCR2-/- monocytes into wild-type recipient animals with zymosan-induced peritonitis. We showed that recruitment of CCR2-/- monocytes was strikingly reduced, by 75%, compared with CCR2+/+ monocytes (Supplementary Figure VI).
Discussion
We report a novel hCD68GFP/ApoE-/- mouse model to quantify macrophage accumulation within atherosclerotic plaques, and to track hCD68GFP+ monocyte recruitment in adoptive transfer studies. High-level monocyte GFP expression, that is sustained upon differentiation into macrophages and foam cells, underpins the ability to perform long-term adoptive transfer and plaque progression/regression studies with this transgenic reporter mouse5. This is in contrast to other myeloid cell reporter lines such as CX3CR1-GFP, which we have previously shown loses GFP fluorescence during macrophage differentiation, and the CD11c YFP mice which show YFP transgene expression that is limited to splenic dendritic cells and alveolar macrophages (Supplementary Figure VII) 5, 7. In contrast, the hCD68GFP mouse shows robust GFP expression in monocytes, macrophages and dendritic cells (Supplementary Figure VII). Analysis of in vitro bone marrow derived macrophages and dendritic cells demonstrated both cell types expressed GFP, with expression of YFP in the CD11cYFP cells providing a much less intense signal in both cell types (Supplementary Figure VIII).
We demonstrate that detection of even rare cells in the artery wall is feasible, given the brightness of GFP expression, even in complex vascular pathologies. The use of either radioactive labeling, bone marrow chimerism, parabiosis or the loading of monocytes with beads (following systemic clodronate liposome administration) have been the primary methods used to directly assess the kinetics of monocyte recruitment to plaque8, 9. However, these methods all have limitations of sensitivity or a requirement for a systemic treatment that may profoundly alter the inflammatory biology of the plaque – such as depletion of endogenous cells or major surgery. In contrast, we can conclusively demonstrate that the GFP+ cells found in plaque were recruited within a 72-hour window showing that monocytes have ongoing and relatively rapid access to the disease tissue. The genetic marking of these cells, rather than bead labeling that can be transferred between cells, will allow studies of the life time and turnover of these cells. Furthermore, the unique advantages of hCD68GFP/ApoE-/- mice will enable studies of single cells for gene expression analysis, without multiple layers of antibody staining or prolonging the time between cell isolation and RNA extraction.
Supplementary Material
Highlights.
The hCD68GFP reporter mouse, crossed with ApoE-/- mice, enables new studies of monocyte recruitment and macrophage accumulation, processes that are critical drivers of atherosclerotic disease.
The high level of GFP fluorescence in circulating monocytes, macrophages and foam cells is sufficient to allow clear discrimination above background in both tissue sections and whole mount en-face aortic preparations, despite the known autofluorescence of these tissues.
hCD68GFP+ monocytes adoptively transferred into mice with atherosclerosis are recruited to both plaque and adjacent adventitia within 72 hours of injection.
Acknowledgements
CD11c-YFP mice were kindly provided by Dr A. Spencer, The Jenner Institute, University of Oxford. Information on the hCD68-GFP mouse can be found at: http://www.cardioscience.ox.ac.uk/hcd68-gfp-transgenic-mouse .
Sources of Funding: This work was supported by the British Heart Foundation [RG/15/10/31485], [RG/12/5/29576] and [FS/12/69/30008], Wellcome Trust [090532/Z/09/Z], and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. KC and EMcN acknowledge support from the BHF Centre of Research Excellence, Oxford [RE/13/1/30181] in supporting Mr Daniel Jones via a summer research studentship.
Abbreviations
- GFP
Green fluorescent protein
- ApoE
Apolioprotein E
- AngII
Angiotensin II
Footnotes
Disclosures: We have no competing financial interests to declare.
References
- 1.Hilgendorf I, Swirski FK, Robbins CS. Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:272–279. doi: 10.1161/ATVBAHA.114.303565. [DOI] [PubMed] [Google Scholar]
- 2.Tang J, Lobatto ME, Hassing L, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Science advances. 2015;1 doi: 10.1126/sciadv.1400223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DE, Stylianou E, McShane H, Channon KM, et al. Human cd68 promoter gfp transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood. 2014;124:e33–44. doi: 10.1182/blood-2014-04-568691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for ccr2 and mcp-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 8.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, et al. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.